FAU Institutional Repository
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1
Pacific Science (1995), vol. 49, no. 2: 143-155 © 1995 by University of Hawai'i Press. All rights reserved Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1 RICHARD EDWARD YOUNG 2 ABSTRACT: Pelagic cephalopods of the mesopelagic-boundary region in Hawai'i have proven difficult to sample but seem to occupy a variety ofhabitats within this zone. Abralia trigonura Berry inhabits the zone only as adults; A. astrosticta Berry may inhabit the inner boundary zone, and Pterygioteuthis giardi Fischer appears to be a facultative inhabitant. Three other mesopelagic species, Liocranchia reinhardti (Steenstrup), Chiroteuthis imperator Chun, and Iridoteuthis iris (Berry), are probable inhabitants; the latter two are suspected to be nonvertical migrants. The mesopelagic-boundary region also contains a variety of other pelagic cephalopods. Some are transients, common species of the mesopelagic zone in offshore waters such as Abraliopsis spp., neritic species such as Euprymna scolopes Berry, and oceanic epipelagic species such as Tremoctopus violaceus Chiaie and Argonauta argo Linnaeus. Others are appar ently permanent but either epipelagic (Onychoteuthis sp. C) or demersal (No totodarus hawaiiensis [Berry] and Haliphron atlanticus Steenstrup). Submersible observations show that Nototodarus hawaiiensis commonly "sits" on the bot tom and Haliphron atlanticus broods its young in the manner of some pelagic octopods. THE CONCEPT OF the mesopelagic-boundary over bottom depths of the same range. The region (m-b region) was first introduced by designation of an inner zone is based on Reid et al. (1991), although a general asso Reid'sfinding mesopelagic fishes resident there ciation of various mesopelagic animals with during both the day and night; mesopelagic land masses has been known for some time. -

Biodiversity of Cephalopod Early-Life Stages Across the Southeastern Brazilian Bight: Spatio-Temporal Patterns in Taxonomic Richness
Marine Biodiversity https://doi.org/10.1007/s12526-019-00980-w ORIGINAL PAPER Biodiversity of cephalopod early-life stages across the Southeastern Brazilian Bight: spatio-temporal patterns in taxonomic richness Carolina C. Araújo1,2 & Maria A. Gasalla1,2 Received: 15 October 2018 /Revised: 20 May 2019 /Accepted: 5 June 2019 # Senckenberg Gesellschaft für Naturforschung 2019 Abstract The diversity patterns of cephalopod early-life stages on the continental shelf of Southeastern Brazilian Bight (SBB, 22–25°S) were investigated using a historical plankton archive of 22 oceanographic cruises carried out from 1974 to 2010. From 874 plankton samples, 438 were positive for cephalopod paralarvae (n = 2116), which were identified to the lowest taxonomic level possible, totaling 15 taxa belonging to 11 families. Richness and diversity indexes (Shannon-Wiener, Simpson, Pielou’seven- ness) revealed a cross-shelf gradient, independent of season and latitude. Abundance k-dominance curves were consistent with this depth-related trend, resulting in high values of k-dominance for the inner shelf during both summer and winter. Two major assemblages were identified by cluster analyses: an inner shelf and a mid-outer shelf. During summer, the inner shelf assemblage was composed of neritic Loliginidae Lesueur, 1821 and epipelagic Argonautidae Tryon, 1879, while in winter, benthic Octopodidae Orbigny, 1840 replaced Argonautidae in importance. These data reveal a remarkable difference in Argonautidae and Octopodidae paralarvae abundance, suggesting a seasonal reproductive pattern for these cephalopods in the SBB. Mesopelagic Enoploteuthidae Pfeffer, 1900 and Ommastrephidae Steenstrup, 1857 characterized the mid-outer shelf assem- blages both in summer and winter. Although based on a higher taxonomic level, the distribution of cephalopod paralarva families reflected not only oceanographic patterns of the SBB but also their adaptations and reproductive strategies. -

Headed Whales (Peponocephala Electra) in Hawaiian Waters
Notes MARINE MAMMAL SCIENCE, 00(00): 00–00 (Month 2018) VC 2018 Society for Marine Mammalogy DOI: 10.1111/mms.12507 Stomach contents and diel diving behavior of melon-headed whales (Peponocephala electra) in Hawaiian waters 1 KRISTI L. WEST, Department of Human Nutrition, Food and Animal Science, College of Tropical Agriculture and Human Resources, Agricultural Sciences 216, 1955 East-West Road, University of Hawai‘i at Manoa, Honolulu, Hawai‘i 96822, U.S.A.; WILLIAM A. WALKER, Marine Mammal Laboratory, National Marine Fisheries Service, NOAA, 7600 Sand Point Way N.E., Seattle, Washington 98115, U.S.A.; ROBIN W. BAIRD,DANIEL L. WEBSTER, and GREGORY S. SCHORR, Cascadia Research Collective, 218 1=2 W. 4th Avenue, Olympia, Washington 98501, U.S.A. Knowledge of the diet and diving behavior of a species is crucial for understand- ing its behavior and ecology, and also has relevance to assessing the impact of poten- tial changes in behavior or spatial use. Assessing diet for many species of cetaceans is difficult, given that most foraging occurs far below the surface and that stomach contents of stranded animals are rarely available. Very little information on food habits or the diving behavior of melon-headed whales (Peponocephala electra)isavail- able from any region of the world. Although there is a paucity of knowledge on melon-headed whales, more is known about them in Hawaiian waters than anywhere else in the world (Aschettino et al. 2012, Woodworth et al. 2012, Baird 2016). In Hawai‘i, two populations of melon-headed whales are recognized, a Hawaiian Islands population estimated to be close to 5,000 individuals that travels offshore and among the islands, and a smaller, inshore population estimated to be about 450 individuals that is found off Hawai‘i Island and known as the Kohala resident population (Aschettino 2010, Aschettino et al. -

Eyes and Extraocular Photoreceptors in Midwater Cephalopods and Fishes: Their Roles in Detecting Downwelling Light for Counterillumination
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by OceanRep Marine Biology 51, 371-380 (1979) MARINE BIOLOGY by Springer-Verlag 1979 Eyes and Extraocular Photoreceptors in Midwater Cephalopods and Fishes: Their Roles in Detecting Downwelling Light for Counterillumination R.E. Young ], C.F.E. Roper 2 and J.F. Waiters3 ]Department of Oceanography, University of Hawaii at Manoa; Honolulu, Hawaii, USA 2Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution; Washington, D.C., USA and 3Maui Community College; Hawaii, USA Abstract The means of detecting downwelling light for counterillumination in several midwa- ter animals has been examined. Eyes and extraocular photoreceptors (dorsal photo- sensitive vesicles in the enoploteuthid squid Abraliopsis sp. B and pineal organs in the myctophid fish Mgctophum spinosum) were alternately exposed to overhead light or covered by a small opaque shield above the animal and the bioluminescent response of the animal was monitored. Covering either the eyes or the extraocular photore- ceptors resulted in a reduction in the intensity of counterillumination. Prelimi- nary experiments examining the bioluminescent feedback mechanism for monitoring intensity of bioluminescence during counterillumination in the midwater squid Abra- lia trigonura indicated that the ventral photosensitive vesicles are responsible for bioluminescent feedback. Introduction range of about 16,OO0-fold which corre- sponds to light changes that occur in Faint but highly directional daylight in clear oceanic waters over a depth range midwaters of the open ocean silhouettes of nearly 300 m (Young et al., in prepa- an opaque animal which could be visible, ration). -
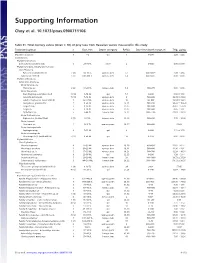
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Giant Protistan Parasites on the Gills of Cephalopods (Mollusca)
DISEASES OF AQUATIC ORGANISMS Vol. 3: 119-125. 1987 Published December 14 Dis. aquat. Org. Giant protistan parasites on the gills of cephalopods (Mollusca) Norman ~c~ean',F. G. ~ochberg~,George L. shinn3 ' Biology Department, San Diego State University, San Diego, California 92182-0057, USA Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta Del Sol Road, Santa Barbara, California 93105. USA Division of Science, Northeast Missouri State University, Kirksville. Missouri 63501, USA ABSTRACT: Large Protista of unknown taxonomic affinities are described from 3 species of coleoid squids, and are reported from many other species of cephalopods. The white to yellow-orange, ovoid cyst-like parasites are partially embedded within small pockets on the surface of the gills, often in large numbers. Except for a holdfast region on one side of the large end, the surface of the parasite is elaborated into low triangular plates separated by grooves. The parasites are uninucleate; their cytoplasm bears lipid droplets and presumed paraglycogen granules. Trichocysts, present in a layer beneath the cytoplasmic surface, were found by transmission electron microscopy to be of the dino- flagellate type. Further studies are needed to clarify the taxonomic position of these protists. INTRODUCTION epoxy resin (see below). One specimen each of the coleoid squids Abralia trigonura and Histioteuthis dof- Cephalopods harbor a diversity of metazoan and leini were trawled near Oahu, Hawaii, in March, 1980. protozoan parasites (Hochberg 1983). In this study we Gill parasites from the former were fixed in formalin; used light and electron microscopy to characterize a those from the latter were fixed in osmium tetroxide. -

Abra/Iopsis Pacificus, a New Species of the Squid Family Enoploteuthidae from the Northwest Pacific
Bull. Natn. Sci. Mus., Tokyo, Ser. A, 16(2), pp. 47-60, June 22, 1990 Abra/iopsis pacificus, a New Species of the Squid Family Enoploteuthidae from the Northwest Pacific By Kotaro TSUCHIYA and Takashi OKUTANI Tokyo University of Fisheries, Tokyo Abstract Abraliopsis (Abraliopsis) pacificus n. sp. is described. This species is char acterized by having diffused photophore arrangement on head, developed carpal flap and aboral keel on tentacular club, unequal offsetcrests on hectocotylus and rectangular arm sucker ring teeth. It is distributed commonly in the mid water of Northwest Pacific Basin. During the systematic study on the Family Enoploteuthidae mainly based on the midwater samples of the R/V Kaiyo-Maru from the Northwest Pacific Ocean, some new facts including undescribed species have been discovered. The previous paper described a new species of the genus Abralia GRAY, 1849, A. similis OKUTANI et Tsu CHIYA, 1987, and here another new species of the genus Abraliopsis JouBIN, 1896 is described. This series of study is aiming at not only describing new taxa of this diverse family, but also making a critical revision of the family Enoploteuthidae. The nominal species of the genus Abraliopsis hitherto known are as follows: Abraliopsis hoylei (PFEFFER, 1884): Western Indian Ocean Abraliopsis lineata (GOODRICH, 1896): Indian Ocean Abraliopsis pfefferi JoUBIN, 1896: Atlantic Abraliopsis ajfinis (PFEFFER, 1912): Eastern Tropical Pacific Abrailopsis gilchristi (ROBSON, 1924): South Africa and New Zealand Abraliopsis felis McGOWAN et OKUTANI, 1968: Northern North Pacific, Transitional Abraliopsis falco YOUNG, 1972: California Current to Northwest Pacific Abraliopsis tui RIDDELL, 1985: New Zealand Abraliopsis chuni NEsrs, 1982: Western Indian Ocean Abraliopsis atlantica NESIS, 1982: Tropical and subtropical Atlantic In addition to this, OKUTANI (1985) has early recognized the existence of an undescribed species of this genus in the Northwest Pacific. -

Factors Affecting Counterillumination As a Cryptic Strategy
Reference: Biol. Bull. 207: 1–16. (August 2004) © 2004 Marine Biological Laboratory Propagation and Perception of Bioluminescence: Factors Affecting Counterillumination as a Cryptic Strategy SO¨ NKE JOHNSEN1,*, EDITH A. WIDDER2, AND CURTIS D. MOBLEY3 1Biology Department, Duke University, Durham, North Carolina 27708; 2Marine Science Division, Harbor Branch Oceanographic Institution, Ft. Pierce, Florida 34946; and 3Sequoia Scientific Inc., Bellevue, Washington 98005 Abstract. Many deep-sea species, particularly crusta- was partially offset by the higher contrast attenuation at ceans, cephalopods, and fish, use photophores to illuminate shallow depths, which reduced the sighting distance of their ventral surfaces and thus disguise their silhouettes mismatches. This research has implications for the study of from predators viewing them from below. This strategy has spatial resolution, contrast sensitivity, and color discrimina- several potential limitations, two of which are examined tion in deep-sea visual systems. here. First, a predator with acute vision may be able to detect the individual photophores on the ventral surface. Introduction Second, a predator may be able to detect any mismatch between the spectrum of the bioluminescence and that of the Counterillumination is a common form of crypsis in the background light. The first limitation was examined by open ocean (Latz, 1995; Harper and Case, 1999; Widder, modeling the perceived images of the counterillumination 1999). Its prevalence is due to the fact that, because the of the squid Abralia veranyi and the myctophid fish Cera- downwelling light is orders of magnitude brighter than the toscopelus maderensis as a function of the distance and upwelling light, even an animal with white ventral colora- visual acuity of the viewer. -

<I>Abralia</I> (Cephalopoda)
BULLETINOF MARINESCIENCE,49(1-2): 113-136, 1991 SQUIDS OF THE GENUS ABRALIA (CEPHALOPODA) FROM THE CENTRAL EQUATORIAL PACIFIC WITH A DESCRIPTION OF ABRALIA HEMINUCHALIS, NEW SPECIES Lourdes Alvina Burgess ABSTRACT Abralia trigonura Berry, 1913, from the Hawaiian Islands is redescribed and a neotype designated. A closely related new species Abralia heminuchalis from the central equatorial Pacific is described. Material from a new area of distribution for Abralia similis Okutani and Tsuchiya, 1987, is discussed. Material for the rare Abralia astrosticta Berry, 1909, is reported, accompanied by a description of a specimen from the Marshall Islands by the late Gilbert L. Voss. All the species examined are illustrated and compared, and observations on immature animals noted. Between 1967 and 1970, while preparing a reference cephalopod collection for the Bureau of Commercial Fisheries Biological Laboratory (now the Honolulu Laboratory, Southwest Center of the National Marine Fisheries Service, National Oceanic and Atmospheric Administration, NOAA), over 5,000 oegopsid squids were identified as belonging to the genus Abralia, family Enoploteuthidae. The four species found were A. trigonura Berry, 1913, A. astrosticta Berry, 1909, and two un-named species then. One of the them has been described as A. simi/is Okutani and Tsuchiya, 1987, the other presented here as Abralia heminuchalis, n. sp. Of the more specimens identified, approximately 1,145 were A. trigonura, 2,367 A, heminuchalis, 1,484 A. similis and 96 A. astrosticta. The samples were taken mainly with a modified Cobb trawl, a 10-foot Isaacs- Kidd trawl, or a Nanaimo trawl during the cruises of several research vessels operated by the laboratory. -

Systematics and Distribution of Abraliopsis (Cephalopoda : Enoploteuthidae) in Australian Waters
University of Wollongong Research Online Faculty of Science, Medicine & Health - Honours Theses University of Wollongong Thesis Collections 2015 Systematics and Distribution of Abraliopsis (Cephalopoda : Enoploteuthidae) in Australian Waters Kwan Wah Li Follow this and additional works at: https://ro.uow.edu.au/thsci University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Li, Kwan Wah, Systematics and Distribution of Abraliopsis (Cephalopoda : Enoploteuthidae) in Australian Waters, BEnviSci Hons, School of Earth & Environmental Sciences, University of Wollongong, 2015. https://ro.uow.edu.au/thsci/113 Research Online is the open access institutional repository for the University of Wollongong. -

<I>Abralia</I> (Cephalopoda: Enoploteuthidae)
BULLETIN OF MARINE SCIENCE, 66(2): 417–443, 2000 NEW SPECIES PAPER SQUIDS OF THE GENUS ABRALIA (CEPHALOPODA: ENOPLOTEUTHIDAE) FROM THE WESTERN TROPICAL PACIFIC WITH A DESCRIPTION OF ABRALIA OMIAE, A NEW SPECIES Kiyotaka Hidaka and Tsunemi Kubodera ABSTRACT Squids of the genus Abralia collected by a midwater trawl in the western tropical Pa- cific were examined. Five species were identified, including Abralia omiae new species. This species is characterized by having five monotypic subocular photophores, two lon- gitudinal broad bands of minute photophores on the ventral mantle, and a hectocotylized right ventral arm with two crests. The other four species identified were A. similis, A. trigonura, A. siedleckyi and A. heminuchalis. Materials from new localities for these spe- cies are discussed and comparisons to related species in several additional characters are made. Squids of the genus Abralia Gray 1849, family Enoploteuthidae, are important mem- bers in the micronektonic communities in the tropical and subtropical world oceans (e.g., Reid et al., 1991). The genus is distinguished in the family Enoploteuthidae by manus of club with one row of hooks and two rows of suckers, absence of enlarged photophores with black coverage on tips of arm IV, buccal crown with typical chromatophores on aboral surface without any other pigmentation, and five to 12 photophores on eyeball (Young et al., 1998). Several systematic studies on pelagic cephalopods in the Pacific Ocean (Quoy and Gaimard, 1832; Berry, 1909, 1913, 1914; Sasaki, 1929; Grimpe, 1931; Nesis and Nikitina, 1987; Okutani and Tsuchiya, 1987; Burgess, 1992) have revealed that nine nominal Abralia species are known from the Pacific.