Metabolic Fingerprinting of Leontopodium Species (Asteraceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protecting the Natural Endangered Heritage in Romania, Croatia, Poland and Slovenia
Available online at http://journals.usamvcluj.ro/index.php/promediu ProEnvironment ProEnvironment 11 (2018) 143-157 Review The Rights of Alive – Protecting the Natural Endangered Heritage in Romania, Croatia, Poland and Slovenia CIOANCĂ Lia-Maria1*, Luminița UJICĂ2, Marijana MIKULANDRA3, Ryszard SOŁTYSIK4, Maja ČERNE5 1Babeș-Bolyai University Cluj-Napoca, University Extension Bistrița, Andrei Mureşanu st., no. 3-5, Romania 2High Scool with Sportive Program Bistrița, Calea Moldovei no. 18. Romania 3OŠ Tina Ujevi Osnovna škola Tina Ujevića Koturaška cesta 75 10000 Zagreb, Croatia 4Zespół Szkół Nr1 w Humniskach, 36 – 206, Huminska 264, Poland 5OŠ Rogaška Slatina, Kidričeva ulica 24, 3250 Rogaška Slatina Slovenia Received 23 July 2018; received and revised form 18 September 2018; accepted 25 September 2018 Available online 30 September 2018 Abstract This article deals with the impact of destructive actions of human population on natural world. As a consequence of relying on non-renewable energy sources and reckless encroachment on natural habitats a lot of plant and animal species have become extinct and more and more species are getting endangered. Thus celebrating biodiversity and solidarity for all life forms, from the tiniest one to the most complex eco-systems, has been in the centre of our attention and operational activities. Keywords: durable development, ecology, endangered species. 1. Introduction Within the massive destruction of forests and forest climate, we witness significant changes, Just as the man has passed from the stage of sometimes radical of the environment. For the animal hunter and collector up to animal raiser and farmer, and plants which have survived through a long period the natural vegetation has increasingly been subject of adaptation, a new difficult era starts again. -

Chrysanthemum Perennials
Perennials & Chrysanthemum North Europe | 2021 INTRO Sehr geehrte Kundinnen Dear customers, und Kunden, im vorliegenden Katalog präsentieren wir unser komplettes Sortiment für den In this catalogue, we are delighted to present our complete autumn assortment. Herbst. Neben den klassischen Stauden, Gräsern und Kräutern haben wir erstmals In addition to the classic perennials, grasses and herbs, we have also included our auch unser attraktives Chrysanthemen Programm integriert. Chrysanthemen sind attractive chrysanthemum range for the first time. Across Europe, chrysanthemums in ganz Europa ein bedeutender Umsatzträger in der zweiten Jahreshälfte. Unsere are major contributors to sales in the second half of the year. Our garden, pot and Garten-, Topf- und Deko-Chrysanthemen sind speziell abgestimmt auf die typischen decorative chrysanthemums are specially adapted to the typical growing conditions Produktionsbedingungen in Nordeuropa und eignen sich mit ihrer Vielfalt an in Northern Europe, and with their wide range of colour combinations are suitable Farbkombinationen für die Vermarktung in allen Absatzkanälen. Einen wesentlichen for marketing across all sales channels. Our extremely successful Autumn Friends Schwerpunkt bildet auch weiterhin unser sehr erfolgreiches Konzept Autumn concept remains a key focus. Tried-and-tested combinations of foliage and flowering Friends. Getestete Kombinationen aus Blatt- und Blütenstauden können zu ver- perennials can be combined to form attractive, ready-to-buy containers, or cultivated kaufsfähigen attraktiven Verkaufsgefäßen zusammengestellt oder direkt als Jung- directly from seedlings. These offers are enthusiastically received by retailers and pflanzen kultiviert werden. Diese Angebote werden von Handel und Verbraucher consumers and will ensure that autumn is a truly joyful time. We wish you a suc- begeistert angenommen und sorgen für Freude im Herbst. -

Switzerland - Alpine Flowers of the Upper Engadine
Switzerland - Alpine Flowers of the Upper Engadine Naturetrek Tour Report 8 - 15 July 2018 Androsace alpina Campanula cochlerariifolia The group at Piz Palu Papaver aurantiacum Report and Images by David Tattersfield Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Switzerland - Alpine Flowers of the Upper Engadine Tour participants: David Tattersfield (leader) with 16 Naturetrek clients Day 1 Sunday 8th July After assembling at Zurich airport, we caught the train to Zurich main station. Once on the intercity express, we settled down to a comfortable journey, through the Swiss countryside, towards the Alps. We passed Lake Zurich and the Walensee, meeting the Rhine as it flows into Liectenstein, and then changed to the UNESCO World Heritage Albula railway at Chur. Dramatic scenery and many loops, tunnels and bridges followed, as we made our way through the Alps. After passing through the long Preda tunnel, we entered a sunny Engadine and made a third change, at Samedan, for the short ride to Pontresina. We transferred to the hotel by minibus and met the remaining two members of our group, before enjoying a lovely evening meal. After a brief talk about the plans for the week, we retired to bed. Day 2 Monday 9th July After a 20-minute walk from the hotel, we caught the 9.06am train at Surovas. We had a scenic introduction to the geography of the region, as we travelled south along the length of Val Bernina, crossing the watershed beside Lago Bianco and alighting at Alp Grum. -

Rock Garden Quarterly
ROCK GARDEN QUARTERLY VOLUME 53 NUMBER 1 WINTER 1995 COVER: Aquilegia scopulorum with vespid wasp by Cindy Nelson-Nold of Lakewood, Colorado All Material Copyright © 1995 North American Rock Garden Society ROCK GARDEN QUARTERLY BULLETIN OF THE NORTH AMERICAN ROCK GARDEN SOCIETY formerly Bulletin of the American Rock Garden Society VOLUME 53 NUMBER 1 WINTER 1995 FEATURES Alpine Gesneriads of Europe, by Darrell Trout 3 Cassiopes and Phyllodoces, by Arthur Dome 17 Plants of Mt. Hutt, a New Zealand Preview, by Ethel Doyle 29 South Africa: Part II, by Panayoti Kelaidis 33 South African Sampler: A Dozen Gems for the Rock Garden, by Panayoti Kelaidis 54 The Vole Story, by Helen Sykes 59 DEPARTMENTS Plant Portrait 62 Books 65 Ramonda nathaliae 2 ROCK GARDEN QUARTERLY VOL. 53:1 ALPINE GESNERIADS OF EUROPE by Darrell Trout J. he Gesneriaceae, or gesneriad Institution and others brings the total family, is a diverse family of mostly Gesneriaceae of China to a count of 56 tropical and subtropical plants with genera and about 413 species. These distribution throughout the world, should provide new horticultural including the north and south temper• material for the rock garden and ate and tropical zones. The 125 genera, alpine house. Yet the choicest plants 2850-plus species include terrestrial for the rock garden or alpine house and epiphytic herbs, shrubs, vines remain the European genera Ramonda, and, rarely, small trees. Botanically, Jancaea, and Haberlea. and in appearance, it is not always easy to separate the family History Gesneriaceae from the closely related The family was named for Konrad Scrophulariaceae (Verbascum, Digitalis, von Gesner, a sixteenth century natu• Calceolaria), the Orobanchaceae, and ralist. -

Die Plantfamilie ASTERACEAE: 6
ISSN 0254-3486 = SA Tydskrif vir Natuurwetenskap en Tegnologie 23, no. 1 & 2 2004 35 Algemene artikel Die plantfamilie ASTERACEAE: 6. Die subfamilie Asteroideae P.P.J. Herman Nasionale Botaniese Instituut, Privaat sak X101, Pretoria, 0001 e-pos: [email protected] UITTREKSEL Die tribusse van die subfamilie Asteroideae word meer volledig in hierdie artikel beskryf. Die genusse wat aan dié tribusse behoort word gelys en hulle verspreiding aangedui. ABSTRACT The plant family Asteraceae: 6. The subfamily Asteroideae. The tribes of the subfamily Asteroideae are described in this article. Genera belonging to the different tribes are listed and their distribution given. INLEIDING Tribus ANTHEMIDEAE Cass. Hierdie artikel is die laaste in die reeks oor die plantfamilie Verteenwoordigers van hierdie tribus is gewoonlik aromaties, Asteraceae.1-5 In die vorige artikel is die klassifikasie bokant byvoorbeeld Artemisia afra (wilde-als), Eriocephalus-soorte, familievlak asook die indeling van die familie Asteraceae in sub- Pentzia-soorte.4 Die feit dat hulle aromaties is, beteken dat hulle families en tribusse bespreek.5 Hierdie artikel handel oor die baie chemiese stowwe bevat. Hierdie stowwe word dikwels subfamilie Asteroideae van die familie Asteraceae, met ’n aangewend vir medisyne (Artemisia) of insekgif (Tanacetum).4 bespreking van die tribusse en die genusse wat aan die verskillende Verder is hulle blaartjies gewoonlik fyn verdeeld en selfs by dié tribusse behoort. Die ‘edelweiss’ wat in die musiekblyspel The met onverdeelde blaartjies, is die blaartjies klein en naaldvormig sound of music besing word, behoort aan die tribus Gnaphalieae (Erica-agtig). Die pappus bestaan gewoonlik uit vry of vergroeide van die subfamilie Asteroideae. -

RHS the Garden Magazine Index 2017
GardenThe INDEX 2017 Volume 142, Parts 1–12 Index 2017 1 January 2017 2 February 2017 3 March 2017 4 April 2017 5 May 2017 6 June 2017 Coloured numbers in Acer: Alchemilla mollis 6: 47, Governor’ 3: 24 in art exhibition, RHS Petheram 4: 31 bold before the page campestre ‘William 48, 49, 51 fanninii 1: 17 Lindley Library 9: 89 Aralia elata ‘Variegata’ 5: number(s) denote the Caldwell’ 8: 41 Alder, Fern, on: Gibbon’s ‘Mistral Tigre’ 10: 7 Newton’s apple tree 2: 31, 31 part number (month). reader’s response Rent alleyway, nemorosa ‘Flore Pleno’ 11 Arbutus unedo 11: 49 Each part is paginated 11: 90 Bermondsey, London 4: 54, 54 ‘Bardsey’ 8: 30 Archer, William separately. cappadocicum 10: 52–55 pavonina 3: 64 ‘Beauty of Bath’ 8: 30 (naturalist) 1: 43 ‘Aureum’ 8: 41 Allium: Angelica sylvestris ‘Braeburn’ 10: 49 arches, plants for 9: Numbers in italics x conspicuum photogram 11: 90 ‘Vicar’s Mead’ 12: 39 ‘Charles Ross’ 8: 30 22–23 denote an image. ‘Phoenix’ 12: 15 atropurpureum 6: 28– Annual General Meeting ‘Devonshire architectural plants 4: 42 davidii ‘Cascade’ 11: 23 29, 29 2017, RHS 1: 67; 7: 93; 9: Quarrenden’ 10: 91 Ardle, Jon, on: Where a plant has a griseum 12: 15, 15, 56, 56 sativum (see garlic) 91 ‘Discovery’ 8: 30, 30 La Seigneurie, Sark 1: Trade Designation micranthum 10: 97, 97 sphaerocephalon 6: 47, Anthriscus sylvestris ‘Gala’ 10: 49 52–56 (also known as a selling palmatum: 50 ‘Ravenswing’ 4: 50, 55 ‘James Grieve’ 8: 30, 30 winter gardening name) it is typeset in ‘Beni-kawa’ 12: 15 triquetrum 8: 15, 15 ants: ‘Katja’ 8: 30 tasks 11: 54–55 a different font to ‘Cascade Gold’ 3: 12, tuberosum flowers as a common black (Lasius ‘Laxton’s Fortune’ 8: Armillaria (see honey distinguish it from the 12 garnish 5: 98, 99, 99 niger) 6: 41 30, 30 fungus, under fungus) cultivar name (shown ‘Sango-kaku’ 12: 15 allotments: on peaches 10: 92 ‘Limelight’ 8: 30 Armitage, James, et al, in ‘Single Quotes’). -

ALFABETISCHE TERMENLIJST Pagina 2 a a Z
ALFABETISCHE TERMENLIJST Pagina 2 A a z. alpha. A = afk. adenine: toegepast in schematische weergave vd. bouw van DNA en RNA. a. = afk. Lat. anno: in het jaar. a-, an- = voorvoegsel met de betekenis: niet, zonder. Å = ångstrom: verouderde lengteeenheid; 1 millimeter is gelijk aan 10 miljoen ångstrom; v. nm, afk. van nanometer. Aalwijn, Aalwee N. ZAfr. = Aloe spp. (Asphodelaceae), ook enkele aloë-achtige verwante soorten. Aaron's Beard N. = Opuntia leucotricha (Cactaceae). Aaron's Rod N. = Koningskaars: Verbascum thapsus (Scrophulariaceae). ABA z. abscisic acid. abaxial ADJ. = aan de vd. as verwijderde zijde, aan de onderzijde (ve. blad); syn. dorsal; ant. adaxial, ventral. abbreviate ADJ. = afgekort. ABC Islands N. = Aruba, Bonaire & Curaçao: de voormalig Ned. eilanden die, tov. de andere Kleine Antillen ver naar het Westen, voor de kust van Venezulela liggen; v. Leeward Islands, Windward Islands. aberrant ADJ. = afwijkend, niet normaal, ongewoon, iets verschilled vh. type; syn. abnormal. abiogenesis N. = veronderstelde ontwikkeling van levende organismen uit dood anorganisch materiaal. abiotic ADJ. = abiotisch: btr. factoren uit de niet-levende omgeving die het leven van planten en dieren beïnvloeden; bv. beschikbaar water, pH vd. bodem, kooldioxidegehalte vd. lucht en licht; v. biotic. abnormal ADJ. = ongewoon, abnormaal, afwijkend; v. aberrant. aboriginal ADJ. = oorspronkelijk, inheems; btr. plant die van nature in een gebied thuis hoort; syn. native, indigeneous; ant. exotic. aborted ADJ. = defect, onvruchtbaar, onvolledig ontwikkeld. abortion N. = het feit dat een orgaan of deel vd. plant zich niet ontwikkelt of in de volwassen plant niet meer aanwezig is. abortive ADJ. = al in een vroeg stadium onvolledig ontwikkeld. Abrojo Sp. N. = 1) Opuntia tunicata (Cactaceae) 2) ook O. -

EDELWEISS and ASTER the Edelweiss and Aster Both Come from the Family of the Sunflower Asteraceae
DN FLOWERS AND PETS Compiled by Damayanthi Hewamanna EDELWEISS and ASTER The Edelweiss and Aster both come from the family of the Sunflower asteraceae delweiss is one of species are used as food Leontopodium-alpinum the well known plants by the young insect of E European mountain a number of Lepidoptera flowers, belonging to the species. Asters can grow in sunflower family. The name all hardiness zones. comes from German edel The genus Aster is now which means noble and generally restricted to the weiss meaning white. Old World species, with Aster in bloom The scientific name, Aster amellus as well as of Leontopodium means the family Asteraceae. The “lion’s paw”, being derived New World species have from the Greek words leon now been reclassified in the meaning lion and podion a genera Almutaster, miniature of pous, meaning Canadanthus, Doellingeria, the foot. plant in many countries, Kingdom : Plantae Eucephalus, Eurybia, Ion- Flowering stalks of Edel- including Mongolia, Bul- actis, Oligoneuron, Ore- weiss can grow to a size of garia, Croatia, Switzerland, (unranked): Angiosperms ostemma, Sericocarpus and 3–20 cm and in cultivation, France, Italy, Germany, (unranked): Eudicots Symphyotrichum, though up to 40 cm). The leaves Spain (Ordesa National (unranked): Asterids all are treated within the appear woolly because of Park), Slovakia (Tatra Order: Asterales tribe Astereae. Regardless the covering of white hairs. National Park), Slovenia (in Family: Asteraceae of the taxonomic change, The flowers are felted and Gorizia and Gradisca since all are still widely referred also woolly with white 1896, in Carniola since Tribe: Gnaphalieae[1] to as Asters in the horticul- hairs, with characteristic 1898), Austria (since 1886) Genus: Leontopodium tural trades. -
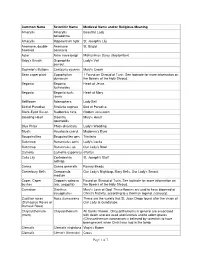
Of 7 Common Name Scientific Name Medieval Name And/Or Religious Meaning Amaryllis Amaryllis Belladonna Beautiful Lady
Common Name Scientific Name Medieval Name and/or Religious Meaning Amaryllis Amaryllis Beautiful Lady belladonna Amaryllis Hippeastrum hybr. St. Joseph's Lily Anemone, double- Anemone St. Brigid flowered coronaria Aster Aster nova-belgii Michaelmas Daisy (September) Baby's Breath Gypsophila Lady's Veil panicul. Bachelor's Buttons Centauria cyannis Mary's Crown Bean caper plant Zygophyllum ? Found on Shroud of Turin. See footnote for more information on dumosum the flowers of the Holy Shroud. Begonia Begonia Heart of Jesus fuchsioides Begonia Begonia fuch. Heart of Mary rosea Bellflower Adenophera Lady Bell Bird of Paradise Streliztia reginae Bird of Paradise Black-Eyed Susan Rudbeckia hirta Golden Jerusalem Bleeding Heart Dicentra Mary's Heart spectabilis Blue Phlox Phlox divaricata Lady's Wedding Bluets Houstonia caerul. Madonna's Eyes Bougainvillea Bougainvillea gen. Trinitaria Buttercup Ranunculus acris Lady's Locks Buttercup Ranunculus sp. Our Lady's Bowl Camelia Camellia (japonica) (Purity) Calla Lily Zantedeshia St. Joseph's Staff aethiop. Canna Canna generalis Rosary Beads Canterbury Bells Campanula Our Lady's Nightcap, Mary Bells, Our Lady's Smock medium Caper, Caper Capparis spinosa Found on Shroud of Turin. See footnote for more information on bushes (var. aegyptia) the flowers of the Holy Shroud. Carnation Dianthus Mary's Love of God. These flowers are said to have bloomed at caryophyllus Christ's Nativity, according to a German legend. (January) Castilian roses Rosa damascena These are the variety that St. Juan Diego found after the vision of (Damascus Roses or Our Lady at Guadalupe. Damask Rose) Chrysanthemum Chrysanthemum All Saints' Flower. Chrysanthemums in general are associated (mum) with death and are used and funerals and to adorn graves (Chrysanthemum coronarium is believed by scientists to have been present when Christ was laid in the tomb. -

Jan Scholten Wonderful Plants Leseprobe Wonderful Plants Von Jan Scholten Herausgeber: Alonnissos Verlag
Jan Scholten Wonderful Plants Leseprobe Wonderful Plants von Jan Scholten Herausgeber: Alonnissos Verlag http://www.narayana-verlag.de/b14446 Im Narayana Webshop finden Sie alle deutschen und englischen Bücher zu Homöopathie, Alternativmedizin und gesunder Lebensweise. Das Kopieren der Leseproben ist nicht gestattet. Narayana Verlag GmbH, Blumenplatz 2, D-79400 Kandern Tel. +49 7626 9749 700 Email [email protected] http://www.narayana-verlag.de 0.1.4 Foreword Lou Klein Hahnemann, trained as a medical translator, researcher and chemist, was at the forefront of science as it was known in his time. In the beginning of homeopathy’s introduction, he led a fervor of pioneering activity and introduced many substances as homeopathic remedies. These were carefully identified and classified as best they could be by the standards of the time, as Hahnemann was a stickler for careful methodologies. Many of his students and followers, such as Hering and Kent, went on to prolifically introduce remedies and clinical concepts in order to advance homeopathy. But as an allopathic “scientific method” took over medicine at the beginning of the 20th century, homeopathy’s growth and momentum lagged. Relative to the time that passed and the developments in science and medicine, minimal evolution and progress in the homeopathic profession was made. There were many reasons for this, notwithstanding the attack on homeopathy from without by allopaths claiming their territory and from within homeopathy where a anachronistic conservative even dogmatically religious ethic took over. Few new homeopathic remedies or techniques were introduced into homeopathy and old systems of classification were relied upon to define and relate what small number of remedies had already been introduced and used. -

Plant Catalog and Sale Information
FREE ADMISSION MAY 11 8 a.m. – 6 p.m. MAY 12 8 a.m. – 5 p.m. PLANT CATALOG AND SALE INFORMATION IT’S YOUR TIME TO GROW! Whether you’re planting a window box, a vegetable garden or a sprawling landscape, we have plants picked just for you. Experts will be onsite to answer your questions and offer advice. Members get a 10% discount on purchases. Don’t Miss the PREVIEW PARTY MAY 10, 4-8 P.M. Enjoy delicious treats, wine and beer as you shop the greatest selection before the sale opens to the public. TICKETS $45 & LIMITED – GET YOURS TODAY! PRESENTING SPONSOR ASSOCIATE SPONSORS 10th & York Street botanicgardens.org TABLE OF CONTENTS ADMISSION & MEMBERSHIP Map 1 Entry to Spring Plant Sale is free on Friday and Saturday. Tickets are required to Annuals 2 attend the Plant Sale Preview Party on Thursday, May 10. Gardens members Aquatics 8 receive 10% off their Spring Plant Sale purchases. New this year: Buy or renew a Container Garden in a Bag 10 membership at the checkout tent when you buy your plants! Fruits, Berries and Vegetables 11 Grown at the Gardens 13 REFUND POLICY Hanging Baskets 14 All products purchased at Spring Plant Sale are non-refundable. Preview Party tickets Herbs 15 cannot be refunded or exchanged. Houseplants 17 Mixed Succulents 18 BRING YOUR WAGON! A limited number of carts will be available. We Perennial Classics 19 highly encourage guests bring their own wagons, ® Plant Select 24 wheelbarrows or carts. Rock Alpine 26 Roses 35 AMENITIES Seeds 36 • Restrooms are located in the lobby of Boettcher Memorial Center, in Marnie’s Pavilion Summer Bulbs 38 and at The Hive Garden Bistro. -

E. Kozuharova, M. Panayotov & V. Spadaro Autecology and Ex Situ
Fl. Medit. 28: 187-206 doi: 10.7320/FlMedit28.187 Version of Record published online on 18 December 2018 E. Kozuharova, M. Panayotov & V. Spadaro Autecology and ex situ growth of Leontopodium nivale subsp. nivale (Asteraceae) from North Pirin marbles (SW Bulgaria) Abstract Kozuharova, E., Panayotov, M. & Spadaro, V.: Autecology and ex situ growth of Leontopodium nivale subsp. nivale (Asteraceae) from North Pirin marbles (SW Bulgaria). — Fl. Medit. 28: 187-206. 2018. — ISSN: 1120-4052 printed, 2240-4538 online. Leontopodium nivale subsp. nivale is a local and disjunct endemic of the central Apennines in Italy and the Pirin Mountains in Bulgaria. The aim of this study is to investigate in situ micro- habitat specifics and ex situ ontogenesis regarding the possible future cultivation and to evalu- ate hazards for wild populations in conditions of human impact and climate change. Leontopodium nivale subsp. nivale is stenobiont which is difficult to grow ex situ and therefore particularly vulnerable. Its wild habitats and populations in Pirin Mts. should be efficiently pro- tected. The results of our study indicate that the stenobiontic plants such as Leontopodium nivale subsp. nivale are particularly subject to hazard. Key words: endemic plant, microhabitat specifics, ex situ ontogenesis, conservation strategy. Introduction Leontopodium (Pers.) R. Br. is a genus of approximately 30 species with an Asian– European disjunct distribution (Blöch & al. 2010). Several molecular clades of Leontopodium, each with morphological integrity have been identified. A distinct one contains the European taxa (Leontopodium alpinum Cass., L. nivale (Ten.) Hand.- Mazz.). These taxa belong to the type section Leontopodium [Alpina Hand.-Mazz.].