Leucogenes Leontopodium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metabolic Fingerprinting of Leontopodium Species (Asteraceae
Phytochemistry 72 (2011) 1379–1389 Contents lists available at ScienceDirect Phytochemistry journal homepage: www.elsevier.com/locate/phytochem Metabolic fingerprinting of Leontopodium species (Asteraceae) by means of 1H NMR and HPLC–ESI-MS Stefan Safer a, Serhat S. Cicek a, Valerio Pieri a, Stefan Schwaiger a, Peter Schneider a, Volker Wissemann b, ⇑ Hermann Stuppner a, a Institute of Pharmacy/Pharmacognosy, Faculty of Chemistry and Pharmacy, Center for Molecular Biosciences Innsbruck (CMBI), University of Innsbruck, Innrain 52c, A-6020 Innsbruck, Austria b Institute of Botany, Systematic Botany Group, Justus-Liebig-University Gießen, Heinrich-Buff-Ring 38, D-35392 Gießen, Germany article info abstract Article history: The genus Leontopodium, mainly distributed in Central and Eastern Asia, consists of ca. 34–58 different Received 30 December 2010 species. The European Leontopodium alpinum, commonly known as Edelweiss, has a long tradition in folk Received in revised form 7 April 2011 medicine. Recent research has resulted in the identification of prior unknown secondary metabolites, Available online 7 May 2011 some of them with interesting biological activities. Despite this, nearly nothing is known about the Asian species of the genus. In this study, we applied proton nuclear magnetic resonance (1H NMR) spectroscopy Keywords: and liquid chromatography–mass spectrometry (LC–MS) metabolic fingerprinting to reveal insights into Leontopodium the metabolic patterns of 11 different Leontopodium species, and to conclude on their taxonomic relation- Asteraceae ship. Principal component analysis (PCA) of 1H NMR fingerprints revealed two species groups. Discrimi- Metabolic fingerprinting 1H NMR nators for these groups were identified as fatty acids and sucrose for group A, and ent-kaurenoic acid and LC–MS derivatives thereof for group B. -

Protecting the Natural Endangered Heritage in Romania, Croatia, Poland and Slovenia
Available online at http://journals.usamvcluj.ro/index.php/promediu ProEnvironment ProEnvironment 11 (2018) 143-157 Review The Rights of Alive – Protecting the Natural Endangered Heritage in Romania, Croatia, Poland and Slovenia CIOANCĂ Lia-Maria1*, Luminița UJICĂ2, Marijana MIKULANDRA3, Ryszard SOŁTYSIK4, Maja ČERNE5 1Babeș-Bolyai University Cluj-Napoca, University Extension Bistrița, Andrei Mureşanu st., no. 3-5, Romania 2High Scool with Sportive Program Bistrița, Calea Moldovei no. 18. Romania 3OŠ Tina Ujevi Osnovna škola Tina Ujevića Koturaška cesta 75 10000 Zagreb, Croatia 4Zespół Szkół Nr1 w Humniskach, 36 – 206, Huminska 264, Poland 5OŠ Rogaška Slatina, Kidričeva ulica 24, 3250 Rogaška Slatina Slovenia Received 23 July 2018; received and revised form 18 September 2018; accepted 25 September 2018 Available online 30 September 2018 Abstract This article deals with the impact of destructive actions of human population on natural world. As a consequence of relying on non-renewable energy sources and reckless encroachment on natural habitats a lot of plant and animal species have become extinct and more and more species are getting endangered. Thus celebrating biodiversity and solidarity for all life forms, from the tiniest one to the most complex eco-systems, has been in the centre of our attention and operational activities. Keywords: durable development, ecology, endangered species. 1. Introduction Within the massive destruction of forests and forest climate, we witness significant changes, Just as the man has passed from the stage of sometimes radical of the environment. For the animal hunter and collector up to animal raiser and farmer, and plants which have survived through a long period the natural vegetation has increasingly been subject of adaptation, a new difficult era starts again. -

Rock Garden Quarterly
ROCK GARDEN QUARTERLY VOLUME 53 NUMBER 1 WINTER 1995 COVER: Aquilegia scopulorum with vespid wasp by Cindy Nelson-Nold of Lakewood, Colorado All Material Copyright © 1995 North American Rock Garden Society ROCK GARDEN QUARTERLY BULLETIN OF THE NORTH AMERICAN ROCK GARDEN SOCIETY formerly Bulletin of the American Rock Garden Society VOLUME 53 NUMBER 1 WINTER 1995 FEATURES Alpine Gesneriads of Europe, by Darrell Trout 3 Cassiopes and Phyllodoces, by Arthur Dome 17 Plants of Mt. Hutt, a New Zealand Preview, by Ethel Doyle 29 South Africa: Part II, by Panayoti Kelaidis 33 South African Sampler: A Dozen Gems for the Rock Garden, by Panayoti Kelaidis 54 The Vole Story, by Helen Sykes 59 DEPARTMENTS Plant Portrait 62 Books 65 Ramonda nathaliae 2 ROCK GARDEN QUARTERLY VOL. 53:1 ALPINE GESNERIADS OF EUROPE by Darrell Trout J. he Gesneriaceae, or gesneriad Institution and others brings the total family, is a diverse family of mostly Gesneriaceae of China to a count of 56 tropical and subtropical plants with genera and about 413 species. These distribution throughout the world, should provide new horticultural including the north and south temper• material for the rock garden and ate and tropical zones. The 125 genera, alpine house. Yet the choicest plants 2850-plus species include terrestrial for the rock garden or alpine house and epiphytic herbs, shrubs, vines remain the European genera Ramonda, and, rarely, small trees. Botanically, Jancaea, and Haberlea. and in appearance, it is not always easy to separate the family History Gesneriaceae from the closely related The family was named for Konrad Scrophulariaceae (Verbascum, Digitalis, von Gesner, a sixteenth century natu• Calceolaria), the Orobanchaceae, and ralist. -

Die Plantfamilie ASTERACEAE: 6
ISSN 0254-3486 = SA Tydskrif vir Natuurwetenskap en Tegnologie 23, no. 1 & 2 2004 35 Algemene artikel Die plantfamilie ASTERACEAE: 6. Die subfamilie Asteroideae P.P.J. Herman Nasionale Botaniese Instituut, Privaat sak X101, Pretoria, 0001 e-pos: [email protected] UITTREKSEL Die tribusse van die subfamilie Asteroideae word meer volledig in hierdie artikel beskryf. Die genusse wat aan dié tribusse behoort word gelys en hulle verspreiding aangedui. ABSTRACT The plant family Asteraceae: 6. The subfamily Asteroideae. The tribes of the subfamily Asteroideae are described in this article. Genera belonging to the different tribes are listed and their distribution given. INLEIDING Tribus ANTHEMIDEAE Cass. Hierdie artikel is die laaste in die reeks oor die plantfamilie Verteenwoordigers van hierdie tribus is gewoonlik aromaties, Asteraceae.1-5 In die vorige artikel is die klassifikasie bokant byvoorbeeld Artemisia afra (wilde-als), Eriocephalus-soorte, familievlak asook die indeling van die familie Asteraceae in sub- Pentzia-soorte.4 Die feit dat hulle aromaties is, beteken dat hulle families en tribusse bespreek.5 Hierdie artikel handel oor die baie chemiese stowwe bevat. Hierdie stowwe word dikwels subfamilie Asteroideae van die familie Asteraceae, met ’n aangewend vir medisyne (Artemisia) of insekgif (Tanacetum).4 bespreking van die tribusse en die genusse wat aan die verskillende Verder is hulle blaartjies gewoonlik fyn verdeeld en selfs by dié tribusse behoort. Die ‘edelweiss’ wat in die musiekblyspel The met onverdeelde blaartjies, is die blaartjies klein en naaldvormig sound of music besing word, behoort aan die tribus Gnaphalieae (Erica-agtig). Die pappus bestaan gewoonlik uit vry of vergroeide van die subfamilie Asteroideae. -

EDELWEISS and ASTER the Edelweiss and Aster Both Come from the Family of the Sunflower Asteraceae
DN FLOWERS AND PETS Compiled by Damayanthi Hewamanna EDELWEISS and ASTER The Edelweiss and Aster both come from the family of the Sunflower asteraceae delweiss is one of species are used as food Leontopodium-alpinum the well known plants by the young insect of E European mountain a number of Lepidoptera flowers, belonging to the species. Asters can grow in sunflower family. The name all hardiness zones. comes from German edel The genus Aster is now which means noble and generally restricted to the weiss meaning white. Old World species, with Aster in bloom The scientific name, Aster amellus as well as of Leontopodium means the family Asteraceae. The “lion’s paw”, being derived New World species have from the Greek words leon now been reclassified in the meaning lion and podion a genera Almutaster, miniature of pous, meaning Canadanthus, Doellingeria, the foot. plant in many countries, Kingdom : Plantae Eucephalus, Eurybia, Ion- Flowering stalks of Edel- including Mongolia, Bul- actis, Oligoneuron, Ore- weiss can grow to a size of garia, Croatia, Switzerland, (unranked): Angiosperms ostemma, Sericocarpus and 3–20 cm and in cultivation, France, Italy, Germany, (unranked): Eudicots Symphyotrichum, though up to 40 cm). The leaves Spain (Ordesa National (unranked): Asterids all are treated within the appear woolly because of Park), Slovakia (Tatra Order: Asterales tribe Astereae. Regardless the covering of white hairs. National Park), Slovenia (in Family: Asteraceae of the taxonomic change, The flowers are felted and Gorizia and Gradisca since all are still widely referred also woolly with white 1896, in Carniola since Tribe: Gnaphalieae[1] to as Asters in the horticul- hairs, with characteristic 1898), Austria (since 1886) Genus: Leontopodium tural trades. -
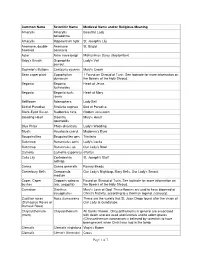
Of 7 Common Name Scientific Name Medieval Name And/Or Religious Meaning Amaryllis Amaryllis Belladonna Beautiful Lady
Common Name Scientific Name Medieval Name and/or Religious Meaning Amaryllis Amaryllis Beautiful Lady belladonna Amaryllis Hippeastrum hybr. St. Joseph's Lily Anemone, double- Anemone St. Brigid flowered coronaria Aster Aster nova-belgii Michaelmas Daisy (September) Baby's Breath Gypsophila Lady's Veil panicul. Bachelor's Buttons Centauria cyannis Mary's Crown Bean caper plant Zygophyllum ? Found on Shroud of Turin. See footnote for more information on dumosum the flowers of the Holy Shroud. Begonia Begonia Heart of Jesus fuchsioides Begonia Begonia fuch. Heart of Mary rosea Bellflower Adenophera Lady Bell Bird of Paradise Streliztia reginae Bird of Paradise Black-Eyed Susan Rudbeckia hirta Golden Jerusalem Bleeding Heart Dicentra Mary's Heart spectabilis Blue Phlox Phlox divaricata Lady's Wedding Bluets Houstonia caerul. Madonna's Eyes Bougainvillea Bougainvillea gen. Trinitaria Buttercup Ranunculus acris Lady's Locks Buttercup Ranunculus sp. Our Lady's Bowl Camelia Camellia (japonica) (Purity) Calla Lily Zantedeshia St. Joseph's Staff aethiop. Canna Canna generalis Rosary Beads Canterbury Bells Campanula Our Lady's Nightcap, Mary Bells, Our Lady's Smock medium Caper, Caper Capparis spinosa Found on Shroud of Turin. See footnote for more information on bushes (var. aegyptia) the flowers of the Holy Shroud. Carnation Dianthus Mary's Love of God. These flowers are said to have bloomed at caryophyllus Christ's Nativity, according to a German legend. (January) Castilian roses Rosa damascena These are the variety that St. Juan Diego found after the vision of (Damascus Roses or Our Lady at Guadalupe. Damask Rose) Chrysanthemum Chrysanthemum All Saints' Flower. Chrysanthemums in general are associated (mum) with death and are used and funerals and to adorn graves (Chrysanthemum coronarium is believed by scientists to have been present when Christ was laid in the tomb. -

E. Kozuharova, M. Panayotov & V. Spadaro Autecology and Ex Situ
Fl. Medit. 28: 187-206 doi: 10.7320/FlMedit28.187 Version of Record published online on 18 December 2018 E. Kozuharova, M. Panayotov & V. Spadaro Autecology and ex situ growth of Leontopodium nivale subsp. nivale (Asteraceae) from North Pirin marbles (SW Bulgaria) Abstract Kozuharova, E., Panayotov, M. & Spadaro, V.: Autecology and ex situ growth of Leontopodium nivale subsp. nivale (Asteraceae) from North Pirin marbles (SW Bulgaria). — Fl. Medit. 28: 187-206. 2018. — ISSN: 1120-4052 printed, 2240-4538 online. Leontopodium nivale subsp. nivale is a local and disjunct endemic of the central Apennines in Italy and the Pirin Mountains in Bulgaria. The aim of this study is to investigate in situ micro- habitat specifics and ex situ ontogenesis regarding the possible future cultivation and to evalu- ate hazards for wild populations in conditions of human impact and climate change. Leontopodium nivale subsp. nivale is stenobiont which is difficult to grow ex situ and therefore particularly vulnerable. Its wild habitats and populations in Pirin Mts. should be efficiently pro- tected. The results of our study indicate that the stenobiontic plants such as Leontopodium nivale subsp. nivale are particularly subject to hazard. Key words: endemic plant, microhabitat specifics, ex situ ontogenesis, conservation strategy. Introduction Leontopodium (Pers.) R. Br. is a genus of approximately 30 species with an Asian– European disjunct distribution (Blöch & al. 2010). Several molecular clades of Leontopodium, each with morphological integrity have been identified. A distinct one contains the European taxa (Leontopodium alpinum Cass., L. nivale (Ten.) Hand.- Mazz.). These taxa belong to the type section Leontopodium [Alpina Hand.-Mazz.]. -

Leontopodium Andersonii (Asteraceae), a New Genus Record for Thailand
THAI FOREST BULL., BOT. 48(1): 21–23. 2020. DOI https://doi.org/10.20531/tfb.2020.48.1.04 Leontopodium andersonii (Asteraceae), a new genus record for Thailand WANNIGA MUNSUK1, PIYAKASET SUKSATHAN2 & PIMWADEE PORNPONGRUNGRUENG1,* ABSTRACT Leontopodium andersonii is here for the first time recorded for Thailand, and is also the first record of the genus Leontopodium (Asteraceae) in Thailand. A description and an illustration are provided. KEYWORDS: Compositae, diversity, Gnaphalieae, taxonomy. Accepted for publication: 7 February 2020. Published online: 21 February 2020 INTRODUCTION MATERIALS AND METHODS During field trips in the mountainous area of Morphological characters were studied using Chiang Mai in 2009, the second author came across a stereo microscope. The measurements were taken an interesting composite with a woolly, silver-white, from dried specimens. For pollen morphology, the star-shaped capitula, which is a distinct characteristic pollen samples were collected and prepared by the of “edelweiss” or Leontopodium, a genus that had acetolysis method (Erdtman, 1960) and observed never been reported in Thailand before. Leontopodium under a light microscope and a Desktop Scanning R.Br. ex Cass. is a monophyletic genus in the tribe Electron Microscopes (MiniSEM) (SNE-4500M). Gnaphalieae (Asteraceae) (Ward et al. 2009; Blöch Pollen description was based on the pollen terminology et al. 2010), characterised by its heterogamous of Walker and Doyle (1975) and Hesse et al. (2009). disciform capitula, in dense or loose terminal corymbs subtended by distinct white lanate bracteal leaves. DESCRIPTION The genus comprises 30–41 species distributed in Asia and Europe with a centre of diversity in the Leontopodium andersonii C.B.Clarke, Compos. -

Flora of China (1994-2013) in English, More Than 100 New Taxa of Chinese Plants Are Still Being Published Each Year
This Book is Sponsored by Shanghai Chenshan Botanical Garden 上海辰山植物园 Shanghai Chenshan Plant Science Research Center, Chinese Academy of Sciences 中国科学院上海辰山植物科学研究中心 Special Fund for Scientific Research of Shanghai Landscaping & City Appearance Administrative Bureau (G182415) 上海市绿化和市容管理局科研专项 (G182415) National Specimen Information Infrastructure, 2018 Special Funds 中国国家标本平台 2018 年度专项 Shanghai Sailing Program (14YF1413800) 上海市青年科技英才扬帆计划 (14YF1413800) Chinese Plant Names Index 2000-2009 DU Cheng & MA Jin-shuang Chinese Plant Names Index 2000-2009 中国植物名称索引 2000-2009 DU Cheng & MA Jin-shuang Abstract The first two volumes of the Chinese Plant Names Index (CPNI) cover the years 2000 through 2009, with entries 1 through 5,516, and 2010 through 2017, with entries 5,517 through 10,795. A unique entry is generated for the specific name of each taxon in a specific publication. Taxonomic treatments cover all novelties at the rank of family, genus, species, subspecies, variety, form and named hybrid taxa, new name changes (new combinations and new names), new records, new synonyms and new typifications for vascular plants reported or recorded from China. Detailed information on the place of publication, including author, publication name, year of publication, volume, issue, and page number, are given in detail. Type specimens and collections information for the taxa and their distribution in China, as well as worldwide, are also provided. The bibliographies were compiled from 182 journals and 138 monographs or books published worldwide. In addition, more than 400 herbaria preserve type specimens of Chinese plants are also listed as an appendix. This book can be used as a basic material for Chinese vascular plant taxonomy, and as a reference for researchers in biodiversity research, environmental protection, forestry and medicinal botany. -

Apomixis in the Asteraceae: Diamonds in the Rough
Functional Plant Science and Biotechnology ©2007 Global Science Books Apomixis in the Asteraceae: Diamonds in the Rough Richard D. Noyes University of Central Arkansas, Conway, Arkansas 72035, USA Correspondence : [email protected] ABSTRACT The Asteraceae is commonly listed, along with Poaceae and Rosaceae, as one of the principal families within which asexual reproduction by seed, i.e., apomixis, is prolific. A review of the literature indicates that naturally occurring apomixis is robustly indicated for 22 genera in seven tribes of Asteraceae (Lactuceae, Gnaphalieae, Astereae, Inuleae, Heliantheae, Madieae, and Eupatorieae), all but one of which occurs in the subfamily Asteroideae. Apomixis has been proposed for an additional 46 genera. However, consideration of the evidence indicates that the trait is contra-indicated for 30 of these cases in which developmental abnormalities or components of apomixis are recorded for otherwise sexual taxa. Accumulation and perpetuation of these reports through generations of reviews has inflated the actual number of genera in which apomictic reproduction occurs in the family. Data are strongly indicative or equivocal for effective apomixis for the remaining 16 genera, but thorough documentation is wanting. Our state of knowledge of apomixis in the Asteraceae is generally poor. Interpreting the phylogenetic distribution and evolution of the trait in the family will require systematic effort involving cytological documentation and genetic analysis of reproduction for many candidate genera. _____________________________________________________________________________________________________________ -

Recent Publications of TOD F
1 Books and Recent Publications of TOD F. STUESSY Books: 1984 Cladistics: Perspectives on the Reconstruction of Evolutionary History. Columbia Univ. Press, N.Y. (edited with T. Duncan). 1985 Cladistic Theory and Methodology. Dowden, Hutchinson and Ross, Philadelphia. (edited with T. Duncan). 1990 Plant Taxonomy: The Systematic Evaluation of Comparative Data. Columbia Univ. Press, N.Y. (Received Gleason Award for 1990). 1994 Case studies in Plant Taxonomy: Exercises in Applied Pattern Recognition. Columbia Univ. Press, N.Y. 1996 Sampling the Green World: Innovative Concepts of Collection, Preservation, and Storage of Plant Diversity. Columbia Univ. Press: New York. (edited with S. Sohmer) 1998 Evolution and Speciation of Island Plants. Cambridge Univ. Press, Cambridge. (edited with M. Ono). 2001 Flavonoids of the Sunflower Family (Asteraceae). Springer-Verlag, Vienna. (with B. Bohm). 2001 Plant Systematics: A Half-Century of Progress (1950-2000) and Future Challenges. IAPT, Vienna. (edited with E Hoerandl and V. Mayer). 2003 Deep Morphology: Toward a Renaissance of Morphology in Plant Systematics. Gantner, Liechtenstein. (edited with V. Mayer and E. Hoerandl). 2009 Plant Taxonomy: The Systematic Evaluation of Comparative Data, ed. 2. Columbia Univ. Press, N.Y. 2009 Systematics, Evolution, and Biogeography of Compositae. IAPT Press, Vienna. (edited with V. Funk, A. Susanna and R. Bayer) (received Stebbins Medal 2009) 2011 Monographic Plant Systematics: Fundamental Assessment of Plant Biodiversity. Gantner, Liechtenstein. (edited with H. Walter Lack) In press Plant Systematics: The Origin, Interpretation, and Ordering of Plant Biodiversity. Gantner, Liechtenstein. (with D. Crawford, D. Soltis, and P. Soltis) Papers (since 2005; more than 280 total): 2005a Pleistocene refugia and recolonization routes in the southern Andes: insights from Hypochaeris palustris (Asteraceae, Lactuceae). -

Multiple Polyploidization Events Across Asteraceae with Two Nested
Multiple Polyploidization Events across Asteraceae with Two Nested Events in the Early History Revealed by Nuclear Phylogenomics Chien-Hsun Huang,1 Caifei Zhang,1 Mian Liu,1 Yi Hu,2 Tiangang Gao,3 Ji Qi,*,1, and Hong Ma*,1 1State Key Laboratory of Genetic Engineering and Collaborative Innovation Center for Genetics and Development, Ministry of Education Key Laboratory of Biodiversity Sciences and Ecological Engineering, Institute of Plant Biology, Institute of Biodiversity Sciences, Center for Evolutionary Biology, School of Life Sciences, Fudan University, Shanghai, China 2Department of Biology, Huck Institutes of the Life Sciences, Pennsylvania State University, State College, PA 3State Key Laboratory of Evolutionary and Systematic Botany, Institute of Botany, the Chinese Academy of Sciences, Beijing, China *Corresponding authors: E-mails: [email protected]; [email protected]. Associate editor: Hideki Innan Abstract Biodiversity results from multiple evolutionary mechanisms, including genetic variation and natural selection. Whole- genome duplications (WGDs), or polyploidizations, provide opportunities for large-scale genetic modifications. Many evolutionarily successful lineages, including angiosperms and vertebrates, are ancient polyploids, suggesting that WGDs are a driving force in evolution. However, this hypothesis is challenged by the observed lower speciation and higher extinction rates of recently formed polyploids than diploids. Asteraceae includes about 10% of angiosperm species, is thus undoubtedly one of the most successful lineages and paleopolyploidization was suggested early in this family using a small number of datasets. Here, we used genes from 64 new transcriptome datasets and others to reconstruct a robust Asteraceae phylogeny, covering 73 species from 18 tribes in six subfamilies. We estimated their divergence times and further identified multiple potential ancient WGDs within several tribes and shared by the Heliantheae alliance, core Asteraceae (Asteroideae–Mutisioideae), and also with the sister family Calyceraceae.