A Detailed Evaluation of the Skeletal Elements of the Skull in the Grey Heron (Ardea Cinerea)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MBB: Head & Neck Anatomy
MBB: Head & Neck Anatomy Skull Osteology • This is a comprehensive guide of all the skull features you must know by the practical exam. • Many of these structures will be presented multiple times during upcoming labs. • This PowerPoint Handout is the resource you will use during lab when you have access to skulls. Mind, Brain & Behavior 2021 Osteology of the Skull Slide Title Slide Number Slide Title Slide Number Ethmoid Slide 3 Paranasal Sinuses Slide 19 Vomer, Nasal Bone, and Inferior Turbinate (Concha) Slide4 Paranasal Sinus Imaging Slide 20 Lacrimal and Palatine Bones Slide 5 Paranasal Sinus Imaging (Sagittal Section) Slide 21 Zygomatic Bone Slide 6 Skull Sutures Slide 22 Frontal Bone Slide 7 Foramen RevieW Slide 23 Mandible Slide 8 Skull Subdivisions Slide 24 Maxilla Slide 9 Sphenoid Bone Slide 10 Skull Subdivisions: Viscerocranium Slide 25 Temporal Bone Slide 11 Skull Subdivisions: Neurocranium Slide 26 Temporal Bone (Continued) Slide 12 Cranial Base: Cranial Fossae Slide 27 Temporal Bone (Middle Ear Cavity and Facial Canal) Slide 13 Skull Development: Intramembranous vs Endochondral Slide 28 Occipital Bone Slide 14 Ossification Structures/Spaces Formed by More Than One Bone Slide 15 Intramembranous Ossification: Fontanelles Slide 29 Structures/Apertures Formed by More Than One Bone Slide 16 Intramembranous Ossification: Craniosynostosis Slide 30 Nasal Septum Slide 17 Endochondral Ossification Slide 31 Infratemporal Fossa & Pterygopalatine Fossa Slide 18 Achondroplasia and Skull Growth Slide 32 Ethmoid • Cribriform plate/foramina -

Splanchnocranium
splanchnocranium - Consists of part of skull that is derived from branchial arches - The facial bones are the bones of the anterior and lower human skull Bones Ethmoid bone Inferior nasal concha Lacrimal bone Maxilla Nasal bone Palatine bone Vomer Zygomatic bone Mandible Ethmoid bone The ethmoid is a single bone, which makes a significant contribution to the middle third of the face. It is located between the lateral wall of the nose and the medial wall of the orbit and forms parts of the nasal septum, roof and lateral wall of the nose, and a considerable part of the medial wall of the orbital cavity. In addition, the ethmoid makes a small contribution to the floor of the anterior cranial fossa. The ethmoid bone can be divided into four parts, the perpendicular plate, the cribriform plate and two ethmoidal labyrinths. Important landmarks include: • Perpendicular plate • Cribriform plate • Crista galli. • Ala. • Ethmoid labyrinths • Medial (nasal) surface. • Orbital plate. • Superior nasal concha. • Middle nasal concha. • Anterior ethmoidal air cells. • Middle ethmoidal air cells. • Posterior ethmoidal air cells. Attachments The falx cerebri (slide) attaches to the posterior border of the crista galli. lamina cribrosa 1 crista galli 2 lamina perpendicularis 3 labyrinthi ethmoidales 4 cellulae ethmoidales anteriores et posteriores 5 lamina orbitalis 6 concha nasalis media 7 processus uncinatus 8 Inferior nasal concha Each inferior nasal concha consists of a curved plate of bone attached to the lateral wall of the nasal cavity. Each consists of inferior and superior borders, medial and lateral surfaces, and anterior and posterior ends. The superior border serves to attach the bone to the lateral wall of the nose, articulating with four different bones. -

Computed Tomographic Assessment of the Lacrimal Sac Fossa in the ଝ
Annals of Anatomy 224 (2019) 23–27 Contents lists available at ScienceDirect Annals of Anatomy jou rnal homepage: www.elsevier.com/locate/aanat RESEARCH ARTICLE Computed tomographic assessment of the lacrimal sac fossa in the ଝ Japanese population a a,∗ a b Tushar Sarbajna , Yasuhiro Takahashi , Ma. Regina Paula Valencia , Makoto Ito , c a Kunihiro Nishimura , Hirohiko Kakizaki a Department of Oculoplastic, Orbital, and Lacrimal Surgery, Aichi Medical University Hospital, Aichi, Japan b Department of Radiology, Aichi Medical University, Aichi, Japan c Department of Otorhinolaryngology, Aichi Medical University, Aichi, Japan a r t i c l e i n f o a b s t r a c t Article history: Purpose: To analyze the morphology of the lacrimal sac fossa in the Japanese population using computed Received 11 February 2019 tomographic images. Received in revised form 25 February 2019 Materials and methods: One-hundred-fifty-five Japanese patients diagnosed with unilateral orbital frac- Accepted 11 March 2019 ture were retrospectively reviewed. Measurements of the dimensions of the lacrimal sac fossa were taken on three anatomical planes (upper, middle, and lower planes) using a digital caliper/protractor tool. Keywords: Results: The mean maximum thickness of the maxillary bone at the upper, middle, and lower planes Lacrimal sac fossa of the lacrimal sac fossa were 4.60 mm, 5.07 mm, and 6.30 mm, respectively. The midpoint thickness of Lacrimal bone the maxillary bone at each plane were 3.04 mm, 3.00 mm, and 2.17 mm, respectively. The lacrimal bone Maxillary bone Japanese thickness at each plane were 1.13 mm, 1.13 mm, and 1.08 mm, respectively. -
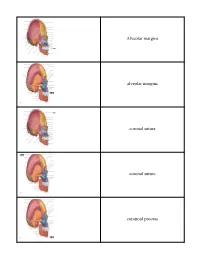
Crista Galli (Part of Cribriform Plate of Ethmoid Bone)
Alveolar margins alveolar margins coronal suture coronal suture coronoid process crista galli (part of cribriform plate of ethmoid bone) ethmoid bone ethmoid bone ethmoid bone external acoustic meatus external occipital crest external occipital protuberance external occipital protuberance frontal bone frontal bone frontal bone frontal sinus frontal squama of frontal bone frontonasal suture glabella incisive fossa inferior nasal concha inferior nuchal line inferior orbital fissure infraorbital foramen internal acoustic meatus lacrimal bone lacrimal bone lacrimal fossa lambdoid suture lambdoid suture lambdoid suture mandible mandible mandible mandibular angle mandibular condyle mandibular foramen mandibular notch mandibular ramus mastoid process of the temporal bone mastoid process of the temporal bone maxilla maxilla maxilla mental foramen mental foramen middle nasal concha of ethmoid bone nasal bone nasal bone nasal bone nasal bone occipital bone occipital bone occipital bone occipitomastoid suture occipitomastoid suture occipitomastoid suture occipital condyle optic canal optic canal palatine bone palatine process of maxilla parietal bone parietal bone parietal bone parietal bone perpendicular plate of ethmoid bone pterygoid process of sphenoid bone sagittal suture sella turcica of sphenoid bone Sphenoid bone (greater wing) spehnoid bone (greater wing) sphenoid bone (greater wing) sphenoid bone (greater wing) sphenoid sinus sphenoid sinus squamous suture squamous suture styloid process of temporal bone superior nuchal line superior orbital fissure supraorbital foramen (notch) supraorbital margin sutural bone temporal bone temporal bone temporal bone vomer bone vomer bone zygomatic bone zygomatic bone. -

Axis Scientific 22-Part Osteopathic Natural Bone Human Skull A-105940
Axis Scientific 22-Part Osteopathic Natural Bone Human Skull A-105940 Frontal Bone Nasal Bone Nasal Bone (Right) (Left) Frontal Bone Ethmoid Bone Parietal Bone Parietal Bone (Right) (Left) Parietal Bone Lacrimal (Right) Bone (Right) Temporal Bone Nasal Bone Temporal Bone (Left) (Right) (Right) Sphenoid Bone Lacrimal Bone (Right) Lacrimal Bone (Left) R L Zygomatic Bone Zygomatic Bone (Right) (Left) Maxilla & Teeth Maxilla & Teeth (Right Side) (Left Side) Inferior Nasal Sphenoid Bone Inferior Nasal Concha (Left) Concha (Right) Occipital Bone Temporal Bone Zygomatic Bone (Right) (Right) Maxilla & Teeth Mandible & Mandible & (Right Side) Vomer Teeth Teeth Anterior View Lateral View Parietal Bone Parietal Bone (Right) (Left) Maxilla & Teeth Maxilla & Teeth (Right Side) (Left Side) Palatine Palatine Bone (Right) Bone (Left) Zygomatic Zygomatic Bone (Left) Bone (Right) Sphenoid Bone R L L R Vomer Temporal Bone Temporal Bone Temporal Bone (Right) (Left) (Right) Temporal Bone (Left) Parietal Bone Parietal Bone (Left) (Right) Occipital Bone Occipital Bone Mandible & Teeth Inferior View Posterior View Parietal Bone Frontal Bone Parietal Bone (Right) (Left) Parietal Bone Parietal Bone (Right) (Left) Temporal Temporal Bone (Right) Bone (Left) Temporal Bone Temporal Bone (Right) (Left) A Frontal Bone R L C B Sphenoid Sphenoid Sphenoid Bone Bone Bone Occipital Bone D E F Zygomatic Bone (Right) Zygomatic G Bone (Left) Lacrimal Bone (Right) Mandible & Maxilla & Teeth Maxilla & Teeth Maxilla & Teeth Teeth (Right Side) (Left Side) Maxilla & Teeth (Left Side) -

New Terminologia Anatomica: Cranium and Extracranial Bones of the Head P.P
Folia Morphol. Vol. 80, No. 3, pp. 477–486 DOI: 10.5603/FM.a2019.0129 R E V I E W A R T I C L E Copyright © 2021 Via Medica ISSN 0015–5659 eISSN 1644–3284 journals.viamedica.pl New Terminologia Anatomica: cranium and extracranial bones of the head P.P. Chmielewski Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University, Wroclaw, Poland [Received: 12 October 2019; Accepted: 17 November 2019; Early publication date: 3 December 2019] In 2019, the updated and extended version of Terminologia Anatomica was published by the Federative International Programme for Anatomical Terminology (FIPAT). This new edition uses more precise and adequate anatomical names compared to its predecessors. Nevertheless, numerous terms have been modified, which poses a challenge to those who prefer traditional anatomical names, i.e. medical students, teachers, clinicians and their instructors. Therefore, there is a need to popularise this new edition of terminology and explain these recent changes. The anatomy of the head, including the cranium, the extracranial bones of the head, the soft parts of the face and the encephalon, poses a particular challenge for medical students but also engenders enthusiasm in those of them who are astute learners. The new version of anatomical terminology concerning the human skull (FIPAT 2019) is presented and briefly discussed in this synopsis. The aim of this article is to present, popularise and explain these interesting modifications that have recently been endorsed by the FIPAT. Based on teaching experience at the Division of Anatomy/Department of Anatomy at Wroclaw Medical University, a brief description of the human skull is given here. -

Endoscopic Dacryocystorh I Nostomy
Endoscopic G. ADRIEN SHUN-SHIN dacryocystorh inostomy: a personal technique The lids are examined and laxity tested. At the biomicroscope, the size of the puncta and Abstract relation to the tear film are noted. The inner Endoscopic dacryocystorhinostomy is a safe, canthus is massaged. Reflux of muco-pus fast and effective method to relieve a stenosis indicates a mucocoele. distal to the common canaliculus. The An updated Jones I test is performed. technique is described in detail with salient Fluorescein is instilled and may demonstrate anatomical points and pre-operative pooling of tears. The nasal cavity is then examination emphasised. inspected. The short 60 mm 00 rigid Hopkins endoscope that fits on an ophthalmoscope Key words Endoscopic dacryocystorhinostomy, handle is the most convenient. If fluorescein is Epiphora, Surgical technique seen at the opening of the duct inferior to the inferior turbinate, then the epiphora is Endoscopic dacryocystorhinostomy (endo DCR) secondary to increased tear production. is gaining in popularity for the relief of a Attention is paid to the size and shape of the stenosis of the nasolacrimal system distal to the nasal cavity as this will determine the ease of common canaliculus. It is the procedure of surgery. A deviated nasal septum or large choice for failed DCR and for some is the first turbinates could require surgery in order to choice as a primary procedure. The relative improve access. The presence of polyps can be merits of surgical endo DCR compared with noted. laser-assisted endo DCR remain to be settled. After a couple of drops of amethocaine, the Here a current personal technique of surgical inferior punctum is dilated with a Nettleship endo DCR will be described. -
Anatomy of the Lacrimal System
1 Anatomy of the Lacrimal System Cat N. Burkat and Mark J. Lucarelli Successful lacrimal surgery begins with a thorough history and preoperative clinical examination, both of which guide the surgeon to the correct diagnosis and appropriate management. A thorough understanding of the anatomy of the lacrimal system will further facilitate the chance of a successful surgical outcome. The following components of the lacrimal drainage system anatomy will be discussed in detail: 1. Embryology 2. Osteology 3. Nasal and paranasal sinuses 4. Secretory system 5. Excretory system Embryology Familiarity with lacrimal system embryology is necessary to under- stand congenital abnormalities of the nasolacrimal drainage system. The orbital walls are embryologically derived from neural crest cells. Ossifi cation of the orbital walls is completed by birth except at the orbital apex. The lesser wing of the sphenoid is initially cartilaginous, unlike the greater wing of the sphenoid and other orbital bones that develop via intramembranous ossifi cation. The membranous bones surrounding the lacrimal excretory system are well developed at 4 months of embryologic age and ossify by birth. The lacrimal gland begins development at the 22- to 25-mm embryo- logic stage as solid epithelial buds arise from the ectoderm of the superolateral conjunctival fornix.1–5 Mesenchymal condensation around these buds forms the secretory lacrimal gland. The early epithelial buds form the orbital lobe in the fi rst 2 months, whereas the secondary buds, which appear later in the 40- to 60-mm stage, develop into the palpebral lobe.1–3 Canalization of the epithelial buds to form ducts occurs, on average, at the 60-mm stage, but may be seen in as early as the 28.5-mm stage.1,3,5 The developing tendon of the levator palpebrae 3 4 C.N. -

Facial Skeleton. Orbit and Nasal Cavity
Facial skeleton. Orbit and nasal cavity. Sándor Katz M.D.,Ph.D. Skull Cerebrocranium= Viscerocranium= Neurocranium Facial skeleton • Frontal bone • Nasal bone • Sphenoid bone • Lacrimal bone • Temporal bone • Ethmoid bone • Parietal bone • Maxilla • Occipital bone • Mandible • Zygomatic bone • Vomer • Palatine bone • Inferior nasal concha • Hyoid bone Viscerocranium= Facial skeleton • Nasal bone • Lacrimal bone • Ethmoid bone • Maxilla • Mandible • Zygomatic bone • Vomer • Palatine bone • Inferior nasal concha • Hyoid bone Nasal bone • internasal septum • piriform aperture Lacrimal bone • posterior lacrimal crest • lacrimal groove • nasolacrimal canal • lacrimal sac Ethmoid bone: perpendicularular plate • crista galli Ethmoid bone: cribriform plate • foramina cribrosa Ethmoid bone: cribriform plate • ethmoidal air cells • ethmoidal labyrinth • orbital (lateral) plate • superior and middle nasal conchae Ethmoid bone: cribriform plate • ethmoid bulla (8) • uncinate process • semilunar hiatus Maxilla: body • infraorbital groove • infraorbital canal • infraorbital foramen • infraorbital margin Maxilla: body • canine fossa Maxilla: body • tuber maxillae • pterygomaxillary fissure • maxillary sinus • maxillary hiatus Maxilla: frontal process • aterior lacrimal crest • piriform aperture zygomatic process Maxilla: alveolar process • alveolar arch • alveolar yokes • anterior nasal spine Maxilla: alveolar process • dental alveolae • interalveolar septa • interradicular septa Maxilla: palatine process • incisive canal • median palatine suture • transverse -

Anatomical and Radiographic Study of the White-Eared Opossum (Didelphis Albiventris) Skull1
Pesq. Vet. Bras. 36(11):1132-1138, novembro 2016 DOI: 10.1590/S0100-736X2016001100013 Anatomical and radiographic study of the white-eared opossum (Didelphis albiventris) skull1 2,4 3 3 2 4 4,5 Bruno C. Schimming *, Luís Felipe F. Reiter , Lívia M. Sandoval , André L. ABSTRACT.-Filadelpho , Letícia R. Inamassu and Maria Jaqueline Mamprim Anatomical and radiographic study of the white-eared opossum (Didelphis albiventris Schimming) skull.B.C., Reiter Pesquisa L.F.F., Veterinária Sandoval BrasileiraL.M., Filadelpho 36(11):1132-1138 A.L., Inamassu L.R. &- Mamprim M.J. 2016. Departa mento de Anatomia, Universidade Estadual Paulista, Cx. Postal 510, Distrito de Rubião Jr s/n, Botucatu, SP 18618-970, Brazil. E-mail: [email protected] This study was made to investigate the anatomical features of the white-eared opossum skull, by osteology and radiographic anatomy. For this, five animals were used without sexual distinction. The skull was examined by radiographic and macroscopic characteristics. The skulls were then subjected to maceration. The skull was described macroscopically according to standard views, i.e. dorsal and caudal, lateral, ventral, and midsagittal. The skull can be- divided into facial (viscerocranium) and cranial (neurocranium) regions. The facial region was elongated and more developed than neurocranium. The supraorbital foramen was ab sent. The tympanic bulla is not well developed. The zygomatic arch was formed by zygomatic process of the temporal bone, zygomatic process of the maxilla, and temporal process of the zygomatic bone. There was no significant difference between bones found in this study when compared with those described for others mammals. -

Surgical Orbital Anatomy
85 Surgical Orbital Anatomy Shirley Hu, MD1,2 Patrick Colley, MD1,2 1 Department of Otolaryngology, Mount Sinai Medical Center, New Address for correspondence Shirley Hu, MD, 310 East 14th Street, York, New York New York, New York 10003 (e-mail: [email protected]). 2 Department of Otolaryngology, New York Eye and Ear Infirmary of MountSinai,NewYork,NewYork Semin Plast Surg 2019;33:85–91. Abstract In this article, the anatomy of the orbit is reviewed, with aspecific emphasis on surgical anatomy. A brief discussion of the ocular globe is also included. The orbits are pyramidal structures separating the upper and middle facial skeletons. The walls, Keywords apex, and base harbor several foramina and fissures as well as bony irregularities where ► orbital anatomy various ligaments, muscles, and capsules attach. There are a variety of surgical ► surgical approaches to the orbit, including the traditional transcutaneous and neurosurgical ► globe techniques and, more recently, minimally invasive, endoscopic approaches. The orbit is a pyramidal structure that encompasses the beyond the inferior orbital fissure, and then gently curves up organ of vision and separates the upper and middle facial toward the superior orbital fissure. When repairing orbital skeletons, with its apex located posteriorly and base situated floor fractures, recreating this subtle curvature will restore anteriorly. The bone comprising the apex and base is much normal anatomyand help prevent malpositioning of the globe.7 thicker than that of the walls, allowing the apex to protect the brain and optic nerve from direct force and the orbital Medial Orbital Wall rim to resist fracture. Pressure to the globe is thus dispersed The medial orbital wall is in the sagittal plane and has the to the curvilinear orbital walls, which serve to maintain the greatest degree of cephalocaudad curvature. -

Patterns of Orbital Disorders
ISSN: 2250-0359 Volume 4 Issue 3.5 2014 Patterns of orbital disorders Balasubramanian Thiagarajan Stanley Medical College Abstract: This article discusses various patterns of presentations of orbital lesions. Since this article has been authored by an otolaryngologist, the entire concept has been viewed from otolaryngologist's angle. With the advent of nasal endoscope trans nasal access to orbit is becoming the order of the day. Major advantage being that external skin incision is avoided. Introduction: The effects caused by orbital diseases are governed by: 1. Pathophysiology of the disease process 2. Anatomic pattern of involvement (location of the lesion). This is more evident from the fact that small tumors of orbital apex causes early symptoms due to involvement of 2,3,4,5 and 6th cranial nerves. These patients will also manifest with progressive vision loss during early course of the lesion. Laterally placed orbital Meningiomas cause features of superior orbital syndrome which could manifest before or along with loss of visual acuity. Anatomy of orbit 1: A careful study of anatomy of orbit is very important to an ENT surgeon because of its proximity to the para nasal sinuses. A comprehensive knowledge of orbital and peri orbital anatomy is necessary to understand the various disorders of this region and in its surgical management. The shape of the orbit resembles a four sided pyramid to begin with but as one goes posterior it becomes three sided towards the apex. The volume of the orbital cavity in an adult is roughly about 30cc. The rim of orbit in an adult measures about 40mm horizontally and 35 mm vertically.