Supporting Information Amézquita Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chromosome Analysis of Five Brazilian
c Indian Academy of Sciences RESEARCH ARTICLE Chromosome analysis of five Brazilian species of poison frogs (Anura: Dendrobatidae) PAULA CAMARGO RODRIGUES1, ODAIR AGUIAR2, FLÁVIA SERPIERI1, ALBERTINA PIMENTEL LIMA3, MASAO UETANEBARO4 and SHIRLEI MARIA RECCO-PIMENTEL1∗ 1Departamento de Anatomia, Biologia Celular e Fisiologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-863 Campinas, São Paulo, Brazil 2Departamento de Biociências, Universidade Federal de São Paulo, Campus Baixada Santista, 11060-001 Santos, São Paulo, Brazil 3Coordenadoria de Pesquisas em Ecologia, Instituto Nacional de Pesquisas do Amazonas, 69011-970 Manaus, Amazonas, Brazil 4Departamento de Biologia, Universidade Federal de Mato Grosso do Sul, 70070-900 Campo Grande, Mato Grosso do Sul, Brazil Abstract Dendrobatid frogs have undergone an extensive systematic reorganization based on recent molecular findings. The present work describes karyotypes of the Brazilian species Adelphobates castaneoticus, A. quinquevittatus, Ameerega picta, A. galactonotus and Dendrobates tinctorius which were compared to each other and with previously described related species. All karyotypes consisted of 2n = 18 chromosomes, except for A. picta which had 2n = 24. The karyotypes of the Adelphobates and D. tinctorius species were highly similar to each other and to the other 2n = 18 previously studied species, revealing conserved karyotypic characteristics in both genera. In recent phylogenetic studies, all Adelphobates species were grouped in a clade separated from the Dendrobates species. Thus, we hypothesized that their common karyotypic traits may have a distinct origin by chromosome rearrangements and mutations. In A. picta, with 2n = 24, chromosome features of pairs from 1 to 8 are shared with other previously karyotyped species within this genus. Hence, the A. -
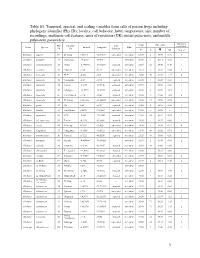
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Reproductive Biology of Ameerega Trivittata(Anura: Dendrobatidae)
ACTA AMAZONICA http://dx.doi.org/10.1590/1809-4392201305384 Reproductive biology of Ameerega trivittata (Anura: Dendrobatidae) in an area of terra firme forest in eastern Amazonia Ellen Cristina Serrão ACIOLI1 * , Selvino NECKEL-OLIVEIRA2 ¹ Universidade Federal do Pará/Museu Paraense Emílio Goeldi, Programa de Pós Graduação em Zoologia, Av. Perimetral 1901/1907, Terra Firme, 66017-970, Belém, Pará, Brasil. ² Universidade Federal de Santa Catarina, Departamento de Ecologia e Zoologia, Centro de Ciências Biológicas, 88040-970, Florianópolis, Santa Catarina, Brasil. * Corresponding author: [email protected] ABSTRACT The reproductive success of tropical amphibians is influenced by factors such as body size and the characteristics of breeding sites. Data on reproductive biology are important for the understanding of population dynamics and the maintenance of species. The objectives of the present study were to examine the abundance ofAmeerega trivittata, analyze the use of microhabitats by calling males and the snout-vent length (SVL) of breeding males and females, the number of tadpoles carried by the males and mature oocytes in the females, as well as the relationship between the SVL of the female and both the number and mean size of the mature oocytes found in the ovaries. Three field trips were conducted between January and September, 2009. A total of 31 plots, with a mean area of 2.3 ha, were surveyed, resulting in records of 235 individuals, with a mean density of 3.26 individuals per hectare. Overall, 66.1% of the individuals sighted were located in the leaf litter, while 17.4% were perched on decaying tree trunks on the forest floor, 15.7% on the aerial roots ofCecropia trees, and 0.8% on lianas. -

Diet Composition of Ameerega Picta
Bonn zoological Bulletin 68 (1): 93–96 ISSN 2190–7307 2019 · Landgref Filho P. et al. http://www.zoologicalbulletin.de https://doi.org/10.20363/BZB-2019.68.1.093 Scientific note urn:lsid:zoobank.org:pub:CBA156F4-E9AA-401C-B036-77C362CE1E89 Diet composition of Ameerega picta (Tschudi, 1838) from the Serra da Bodoquena region in central Brazil, with a summary of dietary studies on species of the genus Ameerega (Anura: Dendrobatidae) Paulo Landgref Filho1, Fabrício H. Oda2, *, Fabio T. Mise3, Domingos de J. Rodrigues4 & Masao Uetanabaro5 1 Universidade Federal de Mato Grosso do Sul, Campus Aquidauana, 79200-000, Aquidauana, Mato Grosso do Sul, Brazil 2 Departamento de Química Biológica, Programa de Pós-graduação em Bioprospecção Molecular, Universidade Regional do Cariri, 63105-00, Crato, Ceará, Brazil 3 Departamento de Biologia, Universidade Estadual do Centro-Oeste, 85040-080, Guarapuava, Paraná, Brazil 4 Instituto de Ciências Naturais, Humanas e Sociais, Universidade Federal de Mato Grosso – Campus Universitário de Sinop, 78557-267, Sinop, Mato Grosso, Brazil 4 Instituto Nacional de Ciência e Tecnologia de Estudos Integrados da Biodiversidade Amazônica – Núcleo Regional de Sinop, Sinop, Mato Grosso, Brazil 5 Rua Clóvis n. 24, 79022-071, Campo Grande, Mato Grosso do Sul, Brazil * Corresponding author: Email: [email protected] 1 urn:lsid:zoobank.org:author:5821C96D-4D9E-4E1D-BE9B-C231B77D1AF3 2 urn:lsid:zoobank.org:author:349712B5-E1B2-4059-A826-58F081DD3A4D 3 urn:lsid:zoobank.org:author:E996AF2A-6CB7-4B73-8DEF-AB7C5EEE1AA0 4 urn:lsid:zoobank.org:author:ACFBA432-3220-4910-AFF5-8FF85053A872 5 urn:lsid:zoobank.org:author:626F6AE3-7D90-4500-BFDC-4E6F4E01B378 Abstract. -

Twenty-Fifth Meeting of the Animals Committee
AC25 Doc. 22 (Rev. 1) Annex 3 (English only / únicamente en inglés / seulement en anglais) Annex 3 Fauna: new species and other changes relating to species listed in the EC wildlife trade regulations – Report compiled by UNEP-WCMC to the European Commission, March, 2011 AC25 Doc. 22 (Rev. 1) Annex 3 – p. 1 Fauna: new species and other taxonomic changes relating to species listed in the EC wildlife trade regulations March, 2011 A report to the European Commission Directorate General E - Environment ENV.E.2. – Environmental Agreements and Trade by the United Nations Environment Programme World Conservation Monitoring Centre AC25 Doc. 22 (Rev. 1) Annex 3 – p. 2 UNEP World Conservation Monitoring Centre 219 Huntingdon Road Cambridge CB3 0DL United Kingdom Tel: +44 (0) 1223 277314 Fax: +44 (0) 1223 277136 Email: [email protected] Website: www.unep-wcmc.org CITATION UNEP-WCMC. 2011. Fauna: new species and other taxonomic changes relating to species ABOUT UNEP-WORLD CONSERVATION listed in the EC wildlife trade regulations. A MONITORING CENTRE report to the European Commission. UNEP- The UNEP World Conservation Monitoring WCMC, Cambridge. Centre (UNEP-WCMC), based in Cambridge, UK, is the specialist biodiversity information and assessment centre of the United Nations Environment Programme (UNEP), run PREPARED FOR cooperatively with WCMC, a UK charity. The The European Commission, Brussels, Belgium Centre's mission is to evaluate and highlight the many values of biodiversity and put authoritative biodiversity knowledge at the DISCLAIMER centre of decision-making. Through the analysis and synthesis of global biodiversity knowledge The contents of this report do not necessarily the Centre provides authoritative, strategic and reflect the views or policies of UNEP or timely information for conventions, countries contributory organisations. -

ANFIBIOS De Cordillera Azul
Parque Nacional Cordillera Azul, San Martín, Loreto, Ucayali y Huánuco, PERÚ 1 ANFIBIOS de Cordillera Azul Kaitlin Tasker y Evan Twomey Fotógrafo indicado debajo de cada foto. Producido por: Tyana Wachter, R. Foster, J. Philipp. Con el apoyo de Connie Keller, el Keller Action Science Center y el Andrew W. Mellon Foundation. © Kaitlin Tasker [[email protected]] y Evan Twomey [[email protected]] y William Duellman [[email protected]] (M): Macho, (H): Hembra, (Juv.): Juvenil © Science and Education, The Field Museum, Chicago [[email protected]] [fieldguides.fieldmuseum.org] Rapid Color Guide # 596 versión 1 10/2015 1 Allobates femoralis 2 Allobates femoralis 3 Allobates ornatus 4 Allobates trilineatus AROMOBATIDAE AROMOBATIDAE AROMOBATIDAE AROMOBATIDAE (foto: K. Tasker) (foto: W. Duellman) (foto: W. Duellman) (foto: W. Duellman) 5 Atelopus pulcher 6 Atelopus pulcher 7 Rhinella cf. festae 8 Rhinella cf. festae BUFONIDAE BUFONIDAE BUFONIDAE BUFONIDAE (foto: K. Tasker) (foto: K. Tasker) (foto: K. Tasker) (foto: K. Tasker) 9 Rhinella cf. festae 10 Rhinella margaritifera 11 Rhinella margaritifera 12 Rhinella marina BUFONIDAE BUFONIDAE BUFONIDAE BUFONIDAE (foto: K. Tasker) (foto: K. Tasker) (foto: K. Tasker) 13 Rhinella marina 14 Rhinella poeppigii 15 Cochranella erminea 16 Hyalinobatrachium cf. carlesvilai BUFONIDAE BUFONIDAE CENTROLENIDAE CENTROLENIDAE (foto: K. Tasker) (foto: W. Duellman) (foto: E. Twomey) (foto: E. Twomey) 17 Hyalinobatrachium pellucidum 18 Hyalinobatrachium pellucidum 19 Hyalinobatrachium pellucidum 20 Nymphargus chancas CENTROLENIDAE CENTROLENIDAE CENTROLENIDAE CENTROLENIDAE (foto: W. Duellman) (foto: W. Duellman) (foto: K. Tasker) (foto: E. Twomey) Parque Nacional Cordillera Azul, San Martín, Loreto, Ucayali y Huánuco, PERÚ 2 ANFIBIOS de Cordillera Azul Kaitlin Tasker y Evan Twomey Fotógrafo indicado debajo de cada foto. -

Does Batrachotoxin Autoresistance Co-Evolve with Toxicity in Phyllobates Poison-Dart Frogs?
Does batrachotoxin autoresistance co-evolve with toxicity in Phyllobates poison-dart frogs? Supplementary material Supplementary Tables Table S1. Specimen information and accession numbers of the sequences used in ancestral sequence reconstructions. For sequences obtained from publicly available genomes or transcriptomes where sequences do not have individual accession numbers (i.e. Nanorana parkeri, Rhinella marina, Rana pipiens) we provide the name of the contig or gene annotation. Species Voucher Locality DI DII DIII DIV Source Observations Allobates femoralis- Lumbaquí, Ecuador KT989177 - - KT989148 Tarvin et al. 2016 Allobates talamancae - Tundaloma, Ecuador KT989178 - - KT989149 Tarvin et al. 2016 Allobates zaparo- Río Pastaza, Ecuador KT989179 - - KT989150 Tarvin et al. 2016 Ameerega bilinguis- Venecia, Ecuador KT989180 KT989205 KT989220 KT989151 Tarvin et al. 2016 Ameerega hahneli- Canelos, Ecuador KT989181 - - KT989152 Tarvin et al. 2016 Ameerega parvula- Gualaquiza, Ecuador KT989182 KT989206 - KT989153 Tarvin et al. 2016 Panguana Biological Ameerega petersi GECOH1407 Station, Puerto Inca, Perú TBA - - TBA This Study Panguana Biological Ameerega picta GECOH1406 Station, Puerto Inca, Perú TBA - - TBA This Study Leticia, Amazonas, Ameerega trivittata TNHC-FS466 Colombia TBA - - TBA This Study La Celia, Risaralda, Andinobates bombetes GECOH1495 Colombia TBA - - TBA This Study Yotoco, Valle del Cauca, Andinobates bombetes GECOH297 Colombia TBA - - TBA This Study Andinobates fulguritus GECOH1314 Lloró, Chocó, Colombia TBA - - TBA This -

Myrmecophagy and Alkaloid Sequestration in Amphibians: a Study on Ameerega Picta (Dendrobatidae) and Elachistocleis Sp
SALAMANDRA 46(1) 11–15 20Myrmecophagy February 2010 and alkaloidISSN 0036–3375 sequestration in amphibians Myrmecophagy and alkaloid sequestration in amphibians: a study on Ameerega picta (Dendrobatidae) and Elachistocleis sp. (Microhylidae) frogs Dietrich Mebs1, Martin Jansen2, Gunther Köhler2, Werner Pogoda1 & Gerold Kauert1 1) Zentrum der Rechtsmedizin, University of Frankfurt, Kennedyallee 104, 60596 Frankfurt, Germany 2) Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Senckenberganlage 25, 60325 Frankfurt, Germany Corresponding author: Dietrich Mebs, e-mail: [email protected] Manuscript received: 16 April 2009 Abstract. Frogs of the family Dendrobatidae and Microhylidae are myrmecophagous, i.e., they feed on ants. It is well accept- ed that toxic alkaloids in the skin secretion of dendrobatid frogs are of dietary origin and may derive from ants. Alcohol ex- tracts from specimens of Ameerega picta (Dendrobatidae) and of Elachistocleis sp. (Microhylidae) from two locations in Bo- livia were analyzed by gas chromatography-mass spectrometry. Whereas the extracts of Ameerega picta specimens exhibited a typical profile of toxic alkaloids, those from Elachistocleis sp. were alkaloid-free. Fecal samples from both frogs consisted of ant remnants. These results indicate that myrmecophagy provides dendrobatids with alkaloids, which they sequester and store in their skin, but an alkaloid sequestering system is absent in the microhylid Elachistocleis sp. Key words. Amphibia, Anura, Dendrobatidae, Microhylidae, myrmecophagy, skin alkaloids. Introduction 996, Biavati et al. 2004). Alkaloids like the pumiliotoxins have been detected in formicine ants of the genera Brachy- Amphibian skin secretions are a rich source of biological- myrmex and Paratrechina (Saporito et al. 2004) as well ly active compounds including a wide variety of chemical as in mites of the family Oribatidae (Takada et al. -

Diversity Within Diversity Parasite Species Richness in Poison Frogs
Molecular Phylogenetics and Evolution 125 (2018) 40–50 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Diversity within diversity: Parasite species richness in poison frogs assessed T by transcriptomics ⁎ Juan C. Santosa, , Rebecca D. Tarvinb, Lauren A. O'Connellc, David C. Blackburnd, Luis A. Colomae,f a Department of Biological Sciences, St. John's University, United States b Integrative Biology, University of Texas at Austin, United States c Department of Biology, Stanford University, United States d Florida Museum of Natural History, United States e Centro Jambatu de Investigación y Conservación de Anfibios, Fundación Otonga, Ecuador f Universidad Regional Amazónica Ikiam, Ecuador ARTICLE INFO ABSTRACT Keywords: Symbionts (e.g., endoparasites and commensals) play an integral role in their host's ecology, yet in many cases Transcriptomics their diversity is likely underestimated. Although endoparasites are traditionally characterized using mor- Co-diversification phology, sequences of conserved genes, and shotgun metagenomics, host transcriptomes constitute an underused Amphibians resource to identify these organisms’ diversity. By isolating non-host transcripts from host transcriptomes, in- Aposematism dividual host tissues can now simultaneously reveal their endoparasite species richness (i.e., number of different Parasitology taxa) and provide insights into parasite gene expression. These approaches can be used in host taxa whose endoparasites are mostly unknown, such as those of tropical amphibians. Here, we focus on the poison frogs (Dendrobatidae) as hosts, which are a Neotropical clade known for their bright coloration and defensive alka- loids. These toxins are an effective protection against vertebrate predators (e.g., snakes and birds), bacteria, and skin-biting ectoparasites (e.g., mosquitoes); however, little is known about their deterrence against eukaryotic endoparasites. -

Vancouver Aquarium's Effort to Save Amphibians
Vancouver Aquarium’s Effort to Save Amphibians Dennis A. Thoney, Ph.D. Darren Smy Kris Rossing Amphibians Are In Trouble 30% - 1,895 of 6,285 amphibians species assessed are threatened with extinction (IUCN) 6% - 382 are near threatened 25% - 1,597 are data deficient Amphibians Are In Trouble 126 species believed to be extinct in wild 39 are extinct in wild but survive in captivity How Are They Disappearing? Chytridiomycosis Habitat destruction Toxicants Introduction of invasive species Climate change Amphibian ARK The IUCN is calling on zoos and aquariums to participate in the global response to this conservation crisis. Recognizing that the rate of decline far outpaces the ability to respond to environmental problems in situ, captive assurance populations have been recognized as the only hope for survival for many amphibian species and will buy time to respond to threats in the wild. The WAZA (World Association of Zoos & Aquariums), IUCN/ CBSG (Conservation Breeding Specialist Group), the IUCN/ASG (Amphibian Specialist Group), and regional zoological associations have hosted a series of workshops and developed a number of resources to support the zoological community’s ex situ response to this crisis. Amphibians Are In Trouble 500 – estimated species whose threats currently cannot be mitigated quickly enough to prevent extinction Each of the 500 largest zoos need to take on at least one species. Amphibians Are In Trouble 500 – estimated species whose threats currently cannot be mitigated quickly enough to prevent extinction Each of the 500 largest zoos need to take on at least one species. 10 managed species in AZA zoos and aquariums 50 extrapolated globally which is only 10% Amphibian Species Maintained by Zoos & Aquariums Globally International Species Information System (ISIS) data base – 800 zoological institutions in 80 countries Number of species maintained (Feb 2012) – 661 species and subspecies maintained. -

Egg Attendance Behaviour of an Amazonian Poison Frog Ameerega Hahneli (Anura: Dendrobatidae)
The Herpetological Bulletin 153, 2020: 31-34 SHORT COMMUNICATION https://doi.org/10.33256/hb153.3134 Egg attendance behaviour of an Amazonian poison frog Ameerega hahneli (Anura: Dendrobatidae) YULFREILER GARAVITO-DAVID1, ESTEFANY CAROLINE GUEVARA-MOLINA2 & JUAN C. DIAZ-RICAURTE1,3,4* 1Semillero de Investigación en Ecofisiología y Biogeografía de Vertebrados, Grupo de investigación en Biodiversidad y Desarrollo Amazónico (BYDA), Programa de Biología, Universidad de la Amazonia, Florencia, Caquetá, Colombia 2Departamento de Fisiologia, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil 3Departamento de Ecologia, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil 4Escola Superior de Agricultura Luiz de Queiroz, Programa de Pós-Graduação em Ecologia Aplicada, Universidade de São Paulo, Piracicaba, Brazil *Corresponding author e-mail: [email protected] he neotropical family Dendrobatidae includes nearly 200 well-documented. However, detailed information on egg Tspecies known as ‘poison frogs’ (Frost, 2020). Poison attendance and parental care is still scarce for most species. frogs lay their eggs in arboreal nests, e.g. leaves on bushes, Here we describe our observations on egg attendance of leaf litter and phytotelmata (Grant et al., 2006; Lötters et al., an Amazonian species of poison frog, Ameerega hahneli 2007; Stynoski et al., 2015). After oviposition, the parents (Boulenger, 1884). attend and take care of the eggs during development until Between 14 and 19 May 2020, we monitored the egg the tadpoles hatch (Weygoldt, 1987; Grant et al., 2006). Once attendance behaviour of a single maleA. hahneli in a relictual they hatch, the adult male or female will typically transport forest in the municipality San José del Fragua, Caquetá, the tadpoles on their backs to a location where each tadpole Colombia (1.352876, -75.971376, WGS84; 466 m a.s.l; Fig.1).