Chromosome Analysis of Five Brazilian
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Article Look but Don't Lick! Find out How These Brightly Colored Frogs
The Power of Poison Look But Don’t Lick! GRADES K-2 NOTES FOR EDUCATORS Common Core State Standards: W.K-2.2, W.K-2.8 Have students read the article “Look but Don’t Lick!” Have them write notes RI.K-2.1, RI.K-2.2, RI.K-2.4, RI.K-2.7, RI.K-2.10 in the large right-hand margin. For example, they could underline key passages, paraphrase important information, or write down questions that New York State Science Core Curriculum: they have. LE 3.1a Next Generation Science Standards: If it is not possible to create color handouts, use a computer projector to PE 1-LS1-2 display the reading so that students can see the colorful frog photos. You DCI LS1.A: Structure and Function may also have them color their black and white copies to match the actual All organisms have external parts. Different colors. animals use their body parts in different ways to see, hear, grasp objects, protect Ask: themselves, move from place to place, and • What does it mean for an animal to be poisonous? (A: An animal is seek, find, and take in food, water and air. poisonous if its body contains a substance that is harmful or fatal to other Plants also have different parts (roots, animals.) stems, leaves, flowers, fruits) that help • How does being poisonous help the frogs in this article survive? (A: them survive and grow. Predators will not eat an animal that is poisonous to them. The frogs signal that they are poisonous by their bright colors, which warn predators not to eat them. -

Effects of Emerging Infectious Diseases on Amphibians: a Review of Experimental Studies
diversity Review Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies Andrew R. Blaustein 1,*, Jenny Urbina 2 ID , Paul W. Snyder 1, Emily Reynolds 2 ID , Trang Dang 1 ID , Jason T. Hoverman 3 ID , Barbara Han 4 ID , Deanna H. Olson 5 ID , Catherine Searle 6 ID and Natalie M. Hambalek 1 1 Department of Integrative Biology, Oregon State University, Corvallis, OR 97331, USA; [email protected] (P.W.S.); [email protected] (T.D.); [email protected] (N.M.H.) 2 Environmental Sciences Graduate Program, Oregon State University, Corvallis, OR 97331, USA; [email protected] (J.U.); [email protected] (E.R.) 3 Department of Forestry and Natural Resources, Purdue University, West Lafayette, IN 47907, USA; [email protected] 4 Cary Institute of Ecosystem Studies, Millbrook, New York, NY 12545, USA; [email protected] 5 US Forest Service, Pacific Northwest Research Station, Corvallis, OR 97331, USA; [email protected] 6 Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA; [email protected] * Correspondence [email protected]; Tel.: +1-541-737-5356 Received: 25 May 2018; Accepted: 27 July 2018; Published: 4 August 2018 Abstract: Numerous factors are contributing to the loss of biodiversity. These include complex effects of multiple abiotic and biotic stressors that may drive population losses. These losses are especially illustrated by amphibians, whose populations are declining worldwide. The causes of amphibian population declines are multifaceted and context-dependent. One major factor affecting amphibian populations is emerging infectious disease. Several pathogens and their associated diseases are especially significant contributors to amphibian population declines. -

Pumiliotoxin Metabolism and Molecular Physiology in a Poison Frog
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.03.367524; this version posted November 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Pumiliotoxin metabolism and molecular physiology in a poison frog 2 3 Aurora Alvarez-Buylla1, Cheyenne Y. Payne1, Charles Vidoudez2, Sunia A. Trauger2 and Lauren 4 A. O’Connell*1 5 6 1 Department of Biology, Stanford University, Stanford, CA 94305, USA 7 2 Harvard Center for Mass Spectrometry, Harvard University, Cambridge, MA 02138, USA 8 9 Running title: Physiology of pumiliotoxin uptake 10 Word count (including methods): 2470 11 Word count (Abstract): 180 12 Key words: alkaloid, cytochrome P450, allopumiliotoxin, RNA sequencing, Dendrobatidae, 13 decahydroquinoline 14 15 * To whom correspondence should be addressed: 16 Lauren A. O’Connell 17 Department of Biology 18 Stanford University 19 371 Jane Stanford Way 20 Stanford, CA 94305 21 [email protected] 22 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.03.367524; this version posted November 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 23 ABSTRACT 24 Poison frogs bioaccumulate alkaloids for chemical defense from their arthropod diet. These 25 small molecules are sequestered from their gastrointestinal tract and transported to the skin for 26 storage. -

National Resource Material Green and Black Poison Frog (Dendrobates Auratus)
Indicative 10 Project National Resource Material Green and Black Poison frog (Dendrobates auratus) Michelle T. Christy and Win Kirkpatrick 2017 Department of Primary Industries and Regional Development 3 Baron-Hay Court, South Perth, WA 6151 An Invasive Animals CRC Project Contents Summary ............................................................................. 2 Key Messages ................................................................... 2 Classification ................................................................... 2 Common names ................................................................ 3 Biology and Ecology ................................................................ 3 Identification ................................................................... 3 Behaviours and Traits ......................................................... 4 Food and Foraging ............................................................. 4 Reproduction and Lifecycle ................................................. 5 Habitat ......................................................................... 5 Global Range ........................................................................ 5 Potential for Introduction ........................................................ 6 Potential for Eradication.......................................................... 7 Impacts ............................................................................... 7 Economic ........................................................................ 7 Environmental -

The Tadpole of Adelphobates Galactonotus (Steindachner, 1864) (Amphibia, Anura, Dendrobatidae)
Zootaxa 4422 (2): 287–290 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Correspondence ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4422.2.8 http://zoobank.org/urn:lsid:zoobank.org:pub:C16E5D1D-D75C-4411-94AE-41C038CA7BA9 The tadpole of Adelphobates galactonotus (Steindachner, 1864) (Amphibia, Anura, Dendrobatidae) DANUSY LOPES SANTOS1,2, 3, SILIONAMÃ PEREIRA DANTAS4 & FAUSTO NOMURA1 1Laboratório de Herpetologia e Comportamento Animal, Departamento de Ecologia, Universidade Federal de Goiás. Campus Samambaia, CEP 74001-970, Goiânia, GO, Brazil 2Laboratório de Ecologia Teórica, Departamento de Zoologia e Botânica, Universidade Estadual Paulista, Campus São José do Rio Preto. CEP 15054-000, São José do Rio Preto, São Paulo, Brazil 4Universidade Federal de Tocantins, Campus Araguaína, CEP 77838-824, Araguaína, TO, Brazil 3Corresponding author. E-mail: [email protected] The systematics of the dart-poison frogs, family Dendrobatidae, experienced several taxonomic rearrangements over time (e.g., Grant et al. 2006, 2017; Brown et al. 2011). Currently, this family comprises 194 described species organized in three sub-families and 15 genera (Frost 2018). Among them, the genus Adelphobates Grant, Frost, Caldwell, Gagliardo, Haddad, Kok, Means, Noonan, Schargel, & Wheeler, 2006, comprises three species, all distributed in Central and lower Amazon drainage of Peru and Brazil, and possibly in northeast of Bolivia (Grant et al. 2006; Frost 2018). Adelphobates galactonotus (Steindachner 1864) is an endemic Brazilian frog, and can be found throughout Pará, Maranhão, Mato Grosso and Tocantins states (Hoogmoed & Avila-Pires 2012), related to Amazon forest formations and also in transitional areas between the Cerrado and the Amazon forest (Valdujo et al. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Advertisement Call of Pratt's Rocket Frog, Colostethus Pratti, from The
SALAMANDRA 56(4): 395–400 SALAMANDRA 30 October 2020 ISSN 0036–3375 Correspondence German Journal of Herpetology Correspondence Advertisement call of Pratt’s rocket frog, Colostethus pratti, from the western Andes of Colombia (Anura: Dendrobatidae) Juan David Jiménez-Bolaño1, Andrés Camilo Montes-Correa1, Franziska Leonhardt2 & Juan Manuel Renjifo1 1) Grupo de Investigación en Manejo & Conservación de Fauna, Flora & Ecosistemas Estratégicos Neotropicales (MIKU), Universidad del Magdalena, Santa Marta, Colombia 2) Museum of Zoology, Senckenberg Natural History Collections Dresden, A. B. Meyer Building, 01109 Dresden, Germany Corresponding author: Andrés Camilo Montes-Correa, e-mail: [email protected] Manuscript received: 22 July 2019 Accepted: 14 April 2020 by Stefan Lötters Northwestern South America is one of the biologically most phibian checklists (Cochran & Goin 1970, Ruiz-Car- diverse yet least known regions of the world. The Tropi- ranza et al. 1994, Acosta-Galvis 2000, Lynch & Suá- cal Andes and Chocó-Tumbes-Magdalena ecoregions, two rez-Mayorga 2004, Ballesteros-Correa et al. 2019). hotspots with high degrees of endemism and conservation The most recent information onC. pratti in Colombia con- priority (Myers et al. 2000, Rodríguez-Mahecha et al. cerns its altitudinal distribution, relative abundance, and 2004), are part of this region. Especially the Chocoan low- natural history in the northernmost part of the western lands and the western Andes of Colombia are areas with Andes, at the Serranía de San Jerónimo, Departamento de exceptional high amphibian diversity and many endemic Córdoba (Romero-Martínez et al. 2008, Ballesteros- taxa (e.g., Lynch et al. 1997, Lynch & Suárez-Mayorga Correa et al. 2019). To the best of our knowledge, there is 2004, Armesto & Señaris 2017). -

Foam Nest Construction and First Report of Agonistic Behaviour In
Neotropical Biology and Conservation 14(1): 117–128 (2019) doi: 10.3897/neotropical.14.e34841 SHORT COMMUNICATION Foam nest construction and first report of agonistic behaviour in Pleurodema tucumanum (Anura, Leptodactylidae) Melina J. Rodriguez Muñoz1,2, Tomás A. Martínez1,2, Juan Carlos Acosta1, Graciela M. Blanco1 1 Gabinete DIBIOVA (Diversidad y Biología de Vertebrados del Árido). Departamento de Biología, FCEFN, Universidad Nacional de San Juan, Avenida Ignacio de la Roza 590, Rivadavia J5400DCS, San Juan, Argentina 2 Consejo Nacional de Investigaciones Científicas y Técnicas, Godoy Cruz 2290, C1425FQB, Ciudad Autónoma de Buenos Aires Argentina Corresponding author: Melina J. Rodriguez Muñoz ([email protected]) Academic editor: A. M. Leal-Zanchet | Received 21 May 2018 | Accepted 27 December 2018 | Published 11 April 2019 Citation: Rodriguez Muñoz MJ, Martínez TA, Acosta JC, Blanco GM (2019) Foam nest construction and first report of agonistic behaviour in Pleurodema tucumanum (Anura: Leptodactylidae). Neotropical Biology and Conservation, 14(1): 117–128. https://doi.org/10.3897/neotropical.14.e34841 Abstract Reproductive strategies are the combination of physiological, morphological, and behavioural traits interacting to increase species reproductive success within a set of environmental conditions. While the reproductive strategies of Leiuperinae are known, few studies have been conducted regarding the reproductive behaviour that underlies them. The aim of this study was to document the structural characteristics of nesting microsites, to describe the process of foam nest construction, and to explore the presence of male agonistic and chorus behaviour in Pleurodema tucumanum. Nests were found close to the edge of a temporary pond and the mean temperature of the foam nests was always close to the mean temperature of the pond water. -
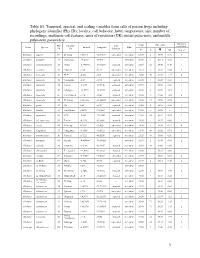
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

Anura: Dendrobatidae: Anomaloglossus) from the Orinoquian Rainforest, Southern Venezuela
TERMS OF USE This pdf is provided by Magnolia Press for private/research use. Commercial sale or deposition in a public library or website is prohibited. Zootaxa 2413: 37–50 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) A new dendrobatid frog (Anura: Dendrobatidae: Anomaloglossus) from the Orinoquian rainforest, southern Venezuela CÉSAR L. BARRIO-AMORÓS1,4, JUAN CARLOS SANTOS2 & OLGA JOVANOVIC3 1,4 Fundación AndígenA, Apartado postal 210, 5101-A Mérida, Venezuela 2University of Texas at Austin, Integrative Biology, 1 University Station C0930 Austin TX 78705, USA 3Division of Evolutionary Biology, Technical University of Braunschweig, Spielmannstr 8, 38106 Braunschweig, Germany 4Corresponding author. E-mail: [email protected]; [email protected] Abstract A new species of Anomaloglossus is described from the Venezuelan Guayana; it is the 21st described species of Anomaloglossus from the Guiana Shield, and the 15th from Venezuela. This species inhabits rainforest on granitic substrate on the northwestern edge of the Guiana Shield (Estado Amazonas, Venezuela). The new species is distinguished from congeners by sexual dimorphism, its unique male color pattern (including two bright orange parotoid marks and two orange paracloacal spots on dark brown background), call characteristics and other morphological features. Though to the new species is known only from the northwestern edge of the Guiana Shield, its distribution may be more extensive, as there are no significant biogeographic barriers isolating the type locality from the granitic lowlands of Venezuela. Key words: Amphibia, Dendrobatidae, Anomaloglossus, Venezuela, Guiana Shield Resumen Se describe una nueva especie de Anomaloglossus de la Guayana venezolana, que es la vigesimoprimera descrita del Escudo Guayanés, y la decimoquinta para Venezuela. -

A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance
Chapter 21 A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance Juan C. Santos , Rebecca D. Tarvin , and Lauren A. O’Connell 21.1 Introduction Chemical defense has evolved multiple times in nearly every major group of life, from snakes and insects to bacteria and plants (Mebs 2002 ). However, among land vertebrates, chemical defenses are restricted to a few monophyletic groups (i.e., clades). Most of these are amphibians and snakes, but a few rare origins (e.g., Pitohui birds) have stimulated research on acquired chemical defenses (Dumbacher et al. 1992 ). Selective pressures that lead to defense are usually associated with an organ- ism’s limited ability to escape predation or conspicuous behaviors and phenotypes that increase detectability by predators (e.g., diurnality or mating calls) (Speed and Ruxton 2005 ). Defended organisms frequently evolve warning signals to advertise their defense, a phenomenon known as aposematism (Mappes et al. 2005 ). Warning signals such as conspicuous coloration unambiguously inform predators that there will be a substantial cost if they proceed with attack or consumption of the defended prey (Mappes et al. 2005 ). However, aposematism is likely more complex than the simple pairing of signal and defense, encompassing a series of traits (i.e., the apose- matic syndrome) that alter morphology, physiology, and behavior (Mappes and J. C. Santos (*) Department of Zoology, Biodiversity Research Centre , University of British Columbia , #4200-6270 University Blvd , Vancouver , BC , Canada , V6T 1Z4 e-mail: [email protected] R. D. Tarvin University of Texas at Austin , 2415 Speedway Stop C0990 , Austin , TX 78712 , USA e-mail: [email protected] L. -

CHKCKLIS I and TAXONO^Irc RIBI JOGRAPHY of the AMPHIBL\NS from PERU
CHKCKLIS I AND TAXONO^irC RIBI JOGRAPHY OF THE AMPHIBL\NS FROM PERU Victor R Morales Asociaci6n de Ecologia y Conservacion/Perii 'MH 2 U 1996 ^JpRARIES SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 107 1995 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles^ We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION Until 1985, when Darrel Frost published the Catalogue of the Amphibians Species of de World, no comprehensive list of amphibians of Peru existed. Now, Rodriguez et al . (1993) have plublished a preliminary list of Amphibians from Peru with species distribution in ecological regions. Herein, I list all the species of amphibians reported from Peru and annotations on some species listed for Rodriguez et al . (op. cit.). The present list contains the following (family/genus/species): in Gymnophiona: 5/6/16, in Caudata: 1/1/3, and in Anura: 9/44/298, the total is 15/51/316.