Homing Behavior in the Neotropical Poison Frog Ameerega Trivittata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Amphibia: Anura: Aromobatidae:Mannophryne
Artículo original / Original article HERPETOTROPICOS 2006 Vol. 3(1):51-57 Copyright © 2007 Univ. Los Andes VARGAS GALARCE and LA MARCA - A NEW SPECIES OF COLLAREDPrinted FROG in Venezuela. All rights reserved51 ISSN 1690-7930 A NEW SPECIES OF COLLARED FROG (AMPHIBIA: ANURA: AROMOBATIDAE: MANNOPHRYNE) FROM THE ANDES OF TRUJILLO STATE, VENEZUELA JÉSSICA Y. VARGAS GALARCE1,2 AND ENRIQUE LA MARCA2,3 1 Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela. 2 Laboratorio de Biogeografía, Escuela de Geografía, Facultad de Ciencias Forestales y Ambientales, Universidad de Los Andes, Mérida, Venezuela. Abstract: We describe a new species of Mannophryne frog, coming from Trujillo State, in the Venezuelan Andes, which constitutes the first species of the genus described from that political unit. This new taxon is distinguished from its congeners by the following combination of characters: small size (SVL males 20.1-23.1 mm, females 23.3-27.5 mm); tympanum about ½ the horizontal length of eye; first finger almost equal, or slightly shorter than second; fingers with thick lateral fringes; tarsal fold conspicuous; foot-web formula: I1.0–0.5II1.0–1.0III1.5–0.5IV0.5–2.0V; toes with lateral flaps; short oblique pale inguinal band; collar moderately wide, solid, with small pale blotches; ventrolateral band absent. We provide data on coloration, in life and in preservative, of specimens of the type series, as well as a description of the larvae and ecological data for the species. Key words: Mannophryne, Amphibia, Anura, Venezuela, Andes, taxonomy, natural history, conservation. Resumen: J.Y. Vargas Galarce y E. -

Anura: Aromobatidae)
bioRxiv preprint doi: https://doi.org/10.1101/771287; this version posted September 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Sexual dichromatism in the neotropical genus Mannophryne (Anura: Aromobatidae) 2 Mark S. Greener1*, Emily Hutton2, Christopher J. Pollock3, Annabeth Wilson2, Chun Yin Lam2, Mohsen 3 Nokhbatolfoghahai 2, Michael J. Jowers4, and J. Roger Downie2 4 1Department of Pathology, Bacteriology and Avian Diseases, Faculty of Veterinary Medicine, Ghent 5 University, B-9820 Merelbeke, Belgium. 6 2 School of Life Sciences, Graham Kerr Building, University of Glasgow, Glasgow G12 8QQ, UK. 7 3 School of Biology, Faculty of Biological sciences, University of Leeds, Leeds LS2 9JT, UK. 8 4 CIBIO/InBIO (Centro de Investigacao em Biodiversidade e Recursos Genticos), Universide do Porto , 9 Vairao 4485-661, Portugal. 10 *[email protected] 11 ABSTRACT 12 Recent reviews on sexual dichromatism in frogs included Mannophryne trinitatis as the only example 13 they could find of dynamic dichromatism (males turn black when calling) within the family 14 Aromobatidae and found no example of ontogenetic dichromatism in this group. We demonstrate 15 ontogenetic dichromatism in M. trinitatis by rearing post-metamorphic froglets to near maturity: the 16 throats of all individuals started as grey coloured; at around seven weeks, the throat became pale 17 yellow in some, and more strongly yellow as development proceeded; the throats of adults are grey 18 in males and variably bright yellow in females, backed by a dark collar. -

Cop18 Doc. 62 (Rev
Original language: Spanish CoP18 Doc. 62 (Rev. 1) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 Species specific matters DRAFT DECISIONS ON THE CONSERVATION OF AMPHIBIANS (AMPHIBIA) 1. This document has been submitted by Costa Rica.* 2. Amphibians are the most threatened class of vertebrates worldwide. According to the IUCN Red List of Threatened Species, "of the 6260 amphibian species assessed, nearly one-third of species (32.4 %) are globally threatened or extinct, representing 2030 species". This is considerably higher than the comparable figure for birds (13 %) or mammals (22 %) (IUCN Red List 2016). The number of species classified as Critically Endangered, Endangered, or Vulnerable has increased throughout the world due to a number of threats, including habitat loss, trade, and disease. While loss of habitat and disease are considered to be the major threats to amphibian populations, harvesting for human use is a further pressure, sometimes posing the greatest threat (IUCN, 2016). 3. Amphibians occur throughout the world, with diversity nuclei in Central America, South America, East and Central Africa, East and South Asia, and Madagascar (Abraham et al. 2013; Jenkins et al. 2013; Pratihar et al. 2014; Pimm et al. 2014, among other authors). Amphibians live in a variety of terrestrial and freshwater ecosystems, ranging from tropical forests to deserts (Stuart et al. 2008). 4. Amphibians have been an important part of human culture for millennia and traditionally served as a source of food (e.g., Mohneke et al. -

Chromosome Analysis of Five Brazilian
c Indian Academy of Sciences RESEARCH ARTICLE Chromosome analysis of five Brazilian species of poison frogs (Anura: Dendrobatidae) PAULA CAMARGO RODRIGUES1, ODAIR AGUIAR2, FLÁVIA SERPIERI1, ALBERTINA PIMENTEL LIMA3, MASAO UETANEBARO4 and SHIRLEI MARIA RECCO-PIMENTEL1∗ 1Departamento de Anatomia, Biologia Celular e Fisiologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-863 Campinas, São Paulo, Brazil 2Departamento de Biociências, Universidade Federal de São Paulo, Campus Baixada Santista, 11060-001 Santos, São Paulo, Brazil 3Coordenadoria de Pesquisas em Ecologia, Instituto Nacional de Pesquisas do Amazonas, 69011-970 Manaus, Amazonas, Brazil 4Departamento de Biologia, Universidade Federal de Mato Grosso do Sul, 70070-900 Campo Grande, Mato Grosso do Sul, Brazil Abstract Dendrobatid frogs have undergone an extensive systematic reorganization based on recent molecular findings. The present work describes karyotypes of the Brazilian species Adelphobates castaneoticus, A. quinquevittatus, Ameerega picta, A. galactonotus and Dendrobates tinctorius which were compared to each other and with previously described related species. All karyotypes consisted of 2n = 18 chromosomes, except for A. picta which had 2n = 24. The karyotypes of the Adelphobates and D. tinctorius species were highly similar to each other and to the other 2n = 18 previously studied species, revealing conserved karyotypic characteristics in both genera. In recent phylogenetic studies, all Adelphobates species were grouped in a clade separated from the Dendrobates species. Thus, we hypothesized that their common karyotypic traits may have a distinct origin by chromosome rearrangements and mutations. In A. picta, with 2n = 24, chromosome features of pairs from 1 to 8 are shared with other previously karyotyped species within this genus. Hence, the A. -
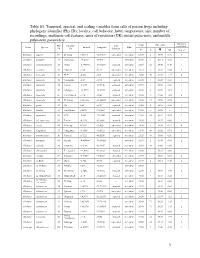
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Reproductive Biology of Ameerega Trivittata(Anura: Dendrobatidae)
ACTA AMAZONICA http://dx.doi.org/10.1590/1809-4392201305384 Reproductive biology of Ameerega trivittata (Anura: Dendrobatidae) in an area of terra firme forest in eastern Amazonia Ellen Cristina Serrão ACIOLI1 * , Selvino NECKEL-OLIVEIRA2 ¹ Universidade Federal do Pará/Museu Paraense Emílio Goeldi, Programa de Pós Graduação em Zoologia, Av. Perimetral 1901/1907, Terra Firme, 66017-970, Belém, Pará, Brasil. ² Universidade Federal de Santa Catarina, Departamento de Ecologia e Zoologia, Centro de Ciências Biológicas, 88040-970, Florianópolis, Santa Catarina, Brasil. * Corresponding author: [email protected] ABSTRACT The reproductive success of tropical amphibians is influenced by factors such as body size and the characteristics of breeding sites. Data on reproductive biology are important for the understanding of population dynamics and the maintenance of species. The objectives of the present study were to examine the abundance ofAmeerega trivittata, analyze the use of microhabitats by calling males and the snout-vent length (SVL) of breeding males and females, the number of tadpoles carried by the males and mature oocytes in the females, as well as the relationship between the SVL of the female and both the number and mean size of the mature oocytes found in the ovaries. Three field trips were conducted between January and September, 2009. A total of 31 plots, with a mean area of 2.3 ha, were surveyed, resulting in records of 235 individuals, with a mean density of 3.26 individuals per hectare. Overall, 66.1% of the individuals sighted were located in the leaf litter, while 17.4% were perched on decaying tree trunks on the forest floor, 15.7% on the aerial roots ofCecropia trees, and 0.8% on lianas. -

The Peculiar Breeding Biology of the Amazonian Frog Allobates Subfolionidificans (Aromobatidae) Anais Da Academia Brasileira De Ciências, Vol
Anais da Academia Brasileira de Ciências ISSN: 0001-3765 [email protected] Academia Brasileira de Ciências Brasil SOUZA, JESUS R.D.; KAEFER, IGOR L.; LIMA, ALBERTINA P. The peculiar breeding biology of the Amazonian frog Allobates subfolionidificans (Aromobatidae) Anais da Academia Brasileira de Ciências, vol. 89, núm. 2, abril-junio, 2017, pp. 885-893 Academia Brasileira de Ciências Rio de Janeiro, Brasil Available in: http://www.redalyc.org/articulo.oa?id=32751197009 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Anais da Academia Brasileira de Ciências (2017) 89(2): 885-893 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201720160245 www.scielo.br/aabc The peculiar breeding biology of the Amazonian frog Allobates subfolionidificans(Aromobatidae) JESUS R.D. SOUZA1, IGOR L. KAEFER2 and ALBERTINA P. LIMA3 1Departamento de Áreas Protegidas e Biodiversidade, Secretaria de Meio Ambiente do Acre, Avenida Benjamin Constant, 856, 69900-062 Rio Branco, AC, Brazil 2Instituto de Ciências Biológicas, Universidade Federal do Amazonas, Avenida Rodrigo Octávio, 6200, 69077-000 Manaus, AM, Brazil 3Coordenação de Pesquisas em Biodiversidade, Instituto Nacional de Pesquisas da Amazônia, Avenida André Araújo, 2936, 69011-970 Manaus, AM, Brazil Manuscript received on April 29, 2016; accepted for publication on August 31, 2016 ABSTRACT Allobates subfolionidificans is a vulnerable and endemic leaf-litter frog from the state of Acre, Brazilian Amazonia. -

The Complete Mitochondrial Genome of Anomaloglossus Baeobatrachus (Amphibia: Anura: Aromobatidae)
MITOCHONDRIAL DNA PART B: RESOURCES, 2016 VOL. 1, NO. 1, 338–340 http://dx.doi.org/10.1080/23802359.2016.1172053 MITOGENOME ANNOUNCEMENT The complete mitochondrial genome of Anomaloglossus baeobatrachus (Amphibia: Anura: Aromobatidae) Jean-Pierre Vachera, Antoine Fouquetb, Hele `ne Holotaa and Christophe Thebaud a aLaboratoire EDB, UMR5174, CNRS-UPS-ENFA, Toulouse, France; bLaboratoire Ecologie, Evolution, Interactions Des Syste`mes Amazoniens (LEEISA), Universite De Guyane, CNRS Guyane, Cayenne, French Guiana ABSTRACT ARTICLE HISTORY The complete mitogenome of the rocket frog Anomaloglossus baeobatrachus was sequenced using a Received 23 March 2016 shotgun approach on an Illumina HiSeq 2500 platform (Illumina Inc., San Diego, CA), providing the first Accepted 25 March 2016 mitogenome for this genus. The genome was 17,572 bp long and presents the typical organization found in other neobatrachian anurans. A phylogenetic analysis including A. baeobatrachus and all other KEYWORDS Amphibia; Aromobatidae; available mitogenomes of Hyloidea provided relationships in accordance with previous phylogenetic Guiana Shield; mitochondrial studies. genome Anomaloglossus baeobatrachus (Boistel & Massary, 1999)isa (Besnard et al. 2014). We obtained a circular sequence of species of frog endemic to the eastern part of the Guiana 17,572 bp in length. The overall base composition was as fol- Shield. It is currently known to occur in French Guiana, lows: A (28.5%), C (27.4%), G (13.9%) and T (30.3). We anno- Suriname and the State of Amapa (Fouquet et al. 2012), and tated the mitogenome with the MITOS webserver (Bernt et al. the State of Para (Avila-Pires et al. 2010). The taxonomy of the 2013). We validated the coding regions using Geneious ver- genus Anomaloglossus is not well resolved, as several mito- sion 9.0.5 (Kearse et al. -

Diet Composition of Ameerega Picta
Bonn zoological Bulletin 68 (1): 93–96 ISSN 2190–7307 2019 · Landgref Filho P. et al. http://www.zoologicalbulletin.de https://doi.org/10.20363/BZB-2019.68.1.093 Scientific note urn:lsid:zoobank.org:pub:CBA156F4-E9AA-401C-B036-77C362CE1E89 Diet composition of Ameerega picta (Tschudi, 1838) from the Serra da Bodoquena region in central Brazil, with a summary of dietary studies on species of the genus Ameerega (Anura: Dendrobatidae) Paulo Landgref Filho1, Fabrício H. Oda2, *, Fabio T. Mise3, Domingos de J. Rodrigues4 & Masao Uetanabaro5 1 Universidade Federal de Mato Grosso do Sul, Campus Aquidauana, 79200-000, Aquidauana, Mato Grosso do Sul, Brazil 2 Departamento de Química Biológica, Programa de Pós-graduação em Bioprospecção Molecular, Universidade Regional do Cariri, 63105-00, Crato, Ceará, Brazil 3 Departamento de Biologia, Universidade Estadual do Centro-Oeste, 85040-080, Guarapuava, Paraná, Brazil 4 Instituto de Ciências Naturais, Humanas e Sociais, Universidade Federal de Mato Grosso – Campus Universitário de Sinop, 78557-267, Sinop, Mato Grosso, Brazil 4 Instituto Nacional de Ciência e Tecnologia de Estudos Integrados da Biodiversidade Amazônica – Núcleo Regional de Sinop, Sinop, Mato Grosso, Brazil 5 Rua Clóvis n. 24, 79022-071, Campo Grande, Mato Grosso do Sul, Brazil * Corresponding author: Email: [email protected] 1 urn:lsid:zoobank.org:author:5821C96D-4D9E-4E1D-BE9B-C231B77D1AF3 2 urn:lsid:zoobank.org:author:349712B5-E1B2-4059-A826-58F081DD3A4D 3 urn:lsid:zoobank.org:author:E996AF2A-6CB7-4B73-8DEF-AB7C5EEE1AA0 4 urn:lsid:zoobank.org:author:ACFBA432-3220-4910-AFF5-8FF85053A872 5 urn:lsid:zoobank.org:author:626F6AE3-7D90-4500-BFDC-4E6F4E01B378 Abstract. -

Anura: Aromobatidae) from the Type Locality (Cachoeira Do Espelho), Xingu River, Brazil
Zootaxa 3475: 86–88 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2012 · Magnolia Press Correspondence ISSN 1175-5334 (online edition) urn:lsid:zoobank.org:pub:D975CEA0-9884-4AF6-BB33-73B76983BABD Advertisement call and colour in life of Allobates crombiei (Morales) “2000” [2002] (Anura: Aromobatidae) from the type Locality (Cachoeira do Espelho), Xingu River, Brazil ALBERTINA P. LIMA1, LUCIANA K. ERDTMANN1 & ADOLFO AMÉZQUITA2 1Coordenação de Biodiversidade, Instituto Nacional de Pesquisas da Amazônia Av. André Araújo, 2936, 69083-000, Manaus, AM, Brasil 2Department of Biological Sciences, Universidad de los Andes, AA 4976, Bogotá, Colombia 1Corresponding author: E-mail: [email protected] Allobates crombiei was described by Morales, “2000” [2002] based on specimens collected by Ronald I. Crombie from Cachoeira do Espelho, on the right bank of the Xingu River, Pará State, Brazil. The original description was short and did not include the call or colour in life. Rodrigues & Caramaschi (2004) suggested that the taxonomic status of this species need be clarified. We are confident that the species collected and recorded by us is Allobates crombiei (Morales) “2000” [2002] because this is the only species of Allobates found calling in forest near Cachoeira do Espelho, and the character diagnosis in preserved specimens is similar, except that, based on preserved specimens, Morales (2002) considered the ventrolateral and the oblique lateral stripes to be absent. This may be because they are imperceptible in preserved specimens. However, unlike recent authors, Morales (2002) also considered the oblique lateral stripe to be absent in Allobates brunneus, Allobates gasconi and Allobates ornatus, in which he illustrated diffuse spots.