Vancouver Aquarium's Effort to Save Amphibians
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fauna of Australia 2A
FAUNA of AUSTRALIA 9. FAMILY MICROHYLIDAE Thomas C. Burton 1 9. FAMILY MICROHYLIDAE Pl 1.3. Cophixalus ornatus (Microhylidae): usually found in leaf litter, this tiny frog is endemic to the wet tropics of northern Queensland. [H. Cogger] 2 9. FAMILY MICROHYLIDAE DEFINITION AND GENERAL DESCRIPTION The Microhylidae is a family of firmisternal frogs, which have broad sacral diapophyses, one or more transverse folds on the surface of the roof of the mouth, and a unique slip to the abdominal musculature, the m. rectus abdominis pars anteroflecta (Burton 1980). All but one of the Australian microhylids are small (snout to vent length less than 35 mm), and all have procoelous vertebrae, are toothless and smooth-bodied, with transverse grooves on the tips of their variously expanded digits. The terminal phalanges of fingers and toes of all Australian microhylids are T-shaped or Y-shaped (Pl. 1.3) with transverse grooves. The Microhylidae consists of eight subfamilies, of which two, the Asterophryinae and Genyophryninae, occur in the Australopapuan region. Only the Genyophryninae occurs in Australia, represented by Cophixalus (11 species) and Sphenophryne (five species). Two newly discovered species of Cophixalus await description (Tyler 1989a). As both genera are also represented in New Guinea, information available from New Guinean species is included in this chapter to remedy deficiencies in knowledge of the Australian fauna. HISTORY OF DISCOVERY The Australian microhylids generally are small, cryptic and tropical, and so it was not until 100 years after European settlement that the first species, Cophixalus ornatus, was collected, in 1888 (Fry 1912). As the microhylids are much more prominent and diverse in New Guinea than in Australia, Australian specimens have been referred to New Guinean species from the time of the early descriptions by Fry (1915), whilst revisions by Parker (1934) and Loveridge (1935) minimised the extent of endemism in Australia. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Sequencing of Mitochondrial DNA with Long Repetitive Regions and Detection of the Frog Lineages of Large Mt Genome Showing Reduction of Purifying Selection
Prime Archives in Genetics: 2nd Edition Book Chapter Sequencing of Mitochondrial DNA with Long Repetitive Regions and Detection of the Frog Lineages of Large mt Genome Showing Reduction of Purifying Selection Ryosuke Kakehashi1, Keitaro Hemmi2, Chiaki Kambayashi1 and Atsushi Kurabayashi1* 1Faculty of Bio-Science, Nagahama Institute of Bio-Science and Technology, Japan 2Amphibian Research Center, Hiroshima University, Japan *Corresponding Author: Atsushi Kurabayashi, Faculty of Bio- Science, Nagahama Institute of Bio-Science and Technology, Japan Published July 12, 2021 This Book Chapter is a republication of an article published by Atsushi Kurabayashi, et al. at International Journal of Genomics in January 2020. (Keitaro Hemmi, Ryosuke Kakehashi, Chiaki Kambayashi, Louis Du Preez, Leslie Minter, Nobuaki Furuno, Atsushi Kurabayashi. Exceptional Enlargement of the Mitochondrial Genome Results from Distinct Causes in Different Rain Frogs (Anura: Brevicipitidae: Breviceps). International Journal of Genomics. Volume 2020, Article ID 6540343, 12 pages. https://doi.org/10.1155/2020/6540343) How to cite this book chapter: Ryosuke Kakehashi, Keitaro Hemmi, Chiaki Kambayashi, Atsushi Kurabayashi. Sequencing of Mitochondrial DNA with Long Repetitive Regions and Detection of the Frog Lineages of Large mt Genome Showing Reduction of Purifying Selection. In: Fekadu Gadissa, editor. Prime Archives in Genetics: 2nd Edition. Hyderabad, India: Vide Leaf. 2021. 1 www.videleaf.com Prime Archives in Genetics: 2nd Edition © The Author(s) 2021. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract The mitochondrial (mt) genome of the bushveld rain frog (Breviceps adspersus, family Brevicipitidae, Afrobatrachia) is the largest (28.8 kbp) among the vertebrates investigated to date. -

Congolius, a New Genus of African Reed Frog Endemic to The
www.nature.com/scientificreports OPEN Congolius, a new genus of African reed frog endemic to the central Congo: A potential case of convergent evolution Tadeáš Nečas1,2*, Gabriel Badjedjea3, Michal Vopálenský4 & Václav Gvoždík1,5* The reed frog genus Hyperolius (Afrobatrachia, Hyperoliidae) is a speciose genus containing over 140 species of mostly small to medium-sized frogs distributed in sub-Saharan Africa. Its high level of colour polymorphism, together with in anurans relatively rare sexual dichromatism, make systematic studies more difcult. As a result, the knowledge of the diversity and taxonomy of this genus is still limited. Hyperolius robustus known only from a handful of localities in rain forests of the central Congo Basin is one of the least known species. Here, we have used molecular methods for the frst time to study the phylogenetic position of this taxon, accompanied by an analysis of phenotype based on external (morphometric) and internal (osteological) morphological characters. Our phylogenetic results undoubtedly placed H. robustus out of Hyperolius into a common clade with sympatric Cryptothylax and West African Morerella. To prevent the uncovered paraphyly, we place H. robustus into a new genus, Congolius. The review of all available data suggests that the new genus is endemic to the central Congolian lowland rain forests. The analysis of phenotype underlined morphological similarity of the new genus to some Hyperolius species. This uniformity of body shape (including cranial shape) indicates that the two genera have either retained ancestral morphology or evolved through convergent evolution under similar ecological pressures in the African rain forests. African reed frogs, Hyperoliidae Laurent, 1943, are presently encompassing almost 230 species in 17 genera. -

Thermal Adaptation of Amphibians in Tropical Mountains
Thermal adaptation of amphibians in tropical mountains. Consequences of global warming Adaptaciones térmicas de anfibios en montañas tropicales: consecuencias del calentamiento global Adaptacions tèrmiques d'amfibis en muntanyes tropicals: conseqüències de l'escalfament global Pol Pintanel Costa ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. -

This Article Appeared in a Journal Published by Elsevier. the Attached
(This is a sample cover image for this issue. The actual cover is not yet available at this time.) This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Toxicon 60 (2012) 967–981 Contents lists available at SciVerse ScienceDirect Toxicon journal homepage: www.elsevier.com/locate/toxicon Antimicrobial peptides and alytesin are co-secreted from the venom of the Midwife toad, Alytes maurus (Alytidae, Anura): Implications for the evolution of frog skin defensive secretions Enrico König a,*, Mei Zhou b, Lei Wang b, Tianbao Chen b, Olaf R.P. Bininda-Emonds a, Chris Shaw b a AG Systematik und Evolutionsbiologie, IBU – Fakultät V, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky Strasse 9-11, 26129 Oldenburg, Germany b Natural Drug Discovery Group, School of Pharmacy, Medical Biology Center, Queen’s University, 97 Lisburn Road, Belfast BT9 7BL, Northern Ireland, UK article info abstract Article history: The skin secretions of frogs and toads (Anura) have long been a known source of a vast Received 23 March 2012 abundance of bioactive substances. -

FROGS in an EFFLUENT SOCIETY Risks, Remedies and Responsibilities by Dr Sara Broomhall First Published in June 2004 by WWF Australia © WWF Australia 2004
FROGS IN AN EFFLUENT SOCIETY Risks, Remedies and Responsibilities by Dr Sara Broomhall First published in June 2004 by WWF Australia © WWF Australia 2004. All Rights Reserved. ISBN: 1 875941 67 3 Author: Dr Sara Broomhall WWF Australia GPO Box 528 Sydney NSW Australia Tel: +612 9281 5515 Fax: +612 9281 1060 www.wwf.org.au For copies of this booklet or a full list of WWF Australia publications on a wide range of conservation issues, please contact us on [email protected] or call 1800 032 551. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of WWF. Special thanks to Craig Cleeland for supplying the photographs for this booklet. CONTENTS FROGS AS ENVIRONMENTAL BAROMETERS The aim of this booklet is to help What is a pollutant? 2 you understand: Australian frogs 2 How do frogs interact with their environment? 3 What pollutants are – Life stages 3 – Habitat requirements 3 How frogs interact with their environment – Ecological position 3 – Frogs and pollutants in the food chain 3 Why water pollution affects frogs Why is environmental pollution a frog issue? 3 – Are frogs more sensitive to environmental pollutants than other species? 3 Where pollutants come from and how they enter the environment WHAT WE DO AND DON’T KNOW Why don’t we have all the answers? 4 How you may be polluting water – How relevant are these toxicity tests to real world situations anyway? 4 Categories of pollutants (such as pesticides) Where do pollutants come from? 4 How many chemicals do we use here in Australia? -

N.Orntates PUBLISHED by the AMERICAN MUSEUM of NATURAL HISTORY CENTRAL PARK WEST at 79TH STREET, NEW YORK, N.Y
AMERICAN MUSEUM N.orntates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 3068, 15 pp., 12 figures, 1 table June 11, 1993 A New Poison Frog from Manu INational Park, Southeastern Peru (Dendrobatidae, Epipedobates) LILY RODRIGUEZ' AND CHARLES W. MYERS2 ABSTRACT Epipedobates macero is a new species of den- ilar to a few other species occurring along the An- drobatid poison frog from lowland rain forest of dean front in eastern Peru, namely E. petersi and the Manu National Park, in the upper Madre de E. cainarachi, which differ in details ofcoloration, Dios drainage ofsoutheastern Peru. It is most sim- morphology, and vocalization. RESUMEN Epipedobates macero, especie nueva, es un den- del llano amaz6nico al pie de los Andes orientales drobatido venenoso de la selva pluvial baja del peruanos, a saber, E. petersi y E. cainarachi, las Parque Nacional del Manu, en el drenaje del Rio cuales difieren en detalles de coloracion, morfo- Alto Madre de Dios, al sudeste del Peru. Es similar logla, y vocalizacion. a otras dos especies que ocurren en los bosques ' Field Associate, Department of Herpetology and Ichthyology, American Museum of Natural History. Investi- gadora Asociada: Asociaci6n Peruana para la Conservaci6n de la Naturaleza (APECO), Parque Jos6 de Acosta 187, Lima 17, Perfi; and Museo de Historia Natural de la Universidad Mayor de San Marcos, apartado 140434, Lima 14, Peru. 2 Curator, Department of Herpetology and Ichthyology, American Museum of Natural History. Copyright © American Museum of Natural History 1993 ISSN 0003-0082 / Price $3.90 2 AMERICAN MUSEUM NOVITATES NO. -

Table 7: Species Changing IUCN Red List Status (2018-2019)
IUCN Red List version 2019-3: Table 7 Last Updated: 10 December 2019 Table 7: Species changing IUCN Red List Status (2018-2019) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2018 (IUCN Red List version 2018-2) and 2019 (IUCN Red List version 2019-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2018) List (2019) change version Category -

3 Translation from Norwegian Regulation on the Import
Translation from Norwegian Regulation on the import, export, re-export and transfer or possession of threatened species of wild flora and fauna (Convention on International Trade in Endangered Species, CITES) Commended by Royal Decree of xx xx 2016 on the authority of the Act of 19 June 2009 no. 100 relating to the Management of Nature Diversity, section 26; the Act of 15 June 2001 no. 79 relating to Environmental Protection on Svalbard, section 26, second paragraph: and the Act of 27 February 1930 no. 2 relating to Jan Mayen, section 2, third paragraph. Commended by Ministry of Climate and Environment. Chapter 1 - Purpose and scope 1. Purpose The purpose of this Regulation is to conserve natural wild species which are, or may become, threatened with extinction as the result of trade. 2. Objective scope This Regulation concerns the import, export and re-export of specimens, alive or dead, of animal and plant species cited in Annex 1. Re-export shall mean export of any specimen that has previously been introduced into the Regulation area. This Regulation also concerns domestic transfer and possession of specimens, alive or dead, of animal and plant species cited in Annex 1. The first and second subparagraphs also concern parts of products that are prepared from or declared as prepared from such species. Hunting trophies are also considered to be dead specimens/ products. Hunting trophy means the whole or recognisable parts of animals, either raw, processed or produced. The first, second and third subparagraphs also concern hybrids. Hybrid means the re-crossing of specimens of species regulated under CITES as far back as the fourth generation, with specimens of species not regulated under CITES. -

Chromosome Analysis of Five Brazilian
c Indian Academy of Sciences RESEARCH ARTICLE Chromosome analysis of five Brazilian species of poison frogs (Anura: Dendrobatidae) PAULA CAMARGO RODRIGUES1, ODAIR AGUIAR2, FLÁVIA SERPIERI1, ALBERTINA PIMENTEL LIMA3, MASAO UETANEBARO4 and SHIRLEI MARIA RECCO-PIMENTEL1∗ 1Departamento de Anatomia, Biologia Celular e Fisiologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-863 Campinas, São Paulo, Brazil 2Departamento de Biociências, Universidade Federal de São Paulo, Campus Baixada Santista, 11060-001 Santos, São Paulo, Brazil 3Coordenadoria de Pesquisas em Ecologia, Instituto Nacional de Pesquisas do Amazonas, 69011-970 Manaus, Amazonas, Brazil 4Departamento de Biologia, Universidade Federal de Mato Grosso do Sul, 70070-900 Campo Grande, Mato Grosso do Sul, Brazil Abstract Dendrobatid frogs have undergone an extensive systematic reorganization based on recent molecular findings. The present work describes karyotypes of the Brazilian species Adelphobates castaneoticus, A. quinquevittatus, Ameerega picta, A. galactonotus and Dendrobates tinctorius which were compared to each other and with previously described related species. All karyotypes consisted of 2n = 18 chromosomes, except for A. picta which had 2n = 24. The karyotypes of the Adelphobates and D. tinctorius species were highly similar to each other and to the other 2n = 18 previously studied species, revealing conserved karyotypic characteristics in both genera. In recent phylogenetic studies, all Adelphobates species were grouped in a clade separated from the Dendrobates species. Thus, we hypothesized that their common karyotypic traits may have a distinct origin by chromosome rearrangements and mutations. In A. picta, with 2n = 24, chromosome features of pairs from 1 to 8 are shared with other previously karyotyped species within this genus. Hence, the A. -
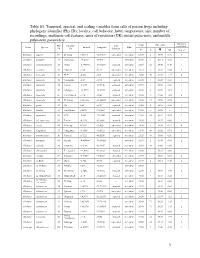
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff.