Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Xenosaurus Tzacualtipantecus. the Zacualtipán Knob-Scaled Lizard Is Endemic to the Sierra Madre Oriental of Eastern Mexico
Xenosaurus tzacualtipantecus. The Zacualtipán knob-scaled lizard is endemic to the Sierra Madre Oriental of eastern Mexico. This medium-large lizard (female holotype measures 188 mm in total length) is known only from the vicinity of the type locality in eastern Hidalgo, at an elevation of 1,900 m in pine-oak forest, and a nearby locality at 2,000 m in northern Veracruz (Woolrich- Piña and Smith 2012). Xenosaurus tzacualtipantecus is thought to belong to the northern clade of the genus, which also contains X. newmanorum and X. platyceps (Bhullar 2011). As with its congeners, X. tzacualtipantecus is an inhabitant of crevices in limestone rocks. This species consumes beetles and lepidopteran larvae and gives birth to living young. The habitat of this lizard in the vicinity of the type locality is being deforested, and people in nearby towns have created an open garbage dump in this area. We determined its EVS as 17, in the middle of the high vulnerability category (see text for explanation), and its status by the IUCN and SEMAR- NAT presently are undetermined. This newly described endemic species is one of nine known species in the monogeneric family Xenosauridae, which is endemic to northern Mesoamerica (Mexico from Tamaulipas to Chiapas and into the montane portions of Alta Verapaz, Guatemala). All but one of these nine species is endemic to Mexico. Photo by Christian Berriozabal-Islas. amphibian-reptile-conservation.org 01 June 2013 | Volume 7 | Number 1 | e61 Copyright: © 2013 Wilson et al. This is an open-access article distributed under the terms of the Creative Com- mons Attribution–NonCommercial–NoDerivs 3.0 Unported License, which permits unrestricted use for non-com- Amphibian & Reptile Conservation 7(1): 1–47. -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Nototriton Nelsoni Is a Moss Salamander Endemic to Cloud Forest in Refugio De Vida Silvestre Texíguat, Located in the Departments of Atlántida and Yoro, Honduras
Nototriton nelsoni is a moss salamander endemic to cloud forest in Refugio de Vida Silvestre Texíguat, located in the departments of Atlántida and Yoro, Honduras. This cryptic species long was confused with N. barbouri, a morphologically similar species now considered endemic to the Sierra de Sulaco in the southern part of the department of Yoro. Like many of its congeners, N. nelsoni rarely is observed in the wild, and is known from just five specimens. Pictured here is the holotype of N. nelsoni, collected above La Liberación in Refugio de Vida Silvestre Texíguat at an elevation of 1,420 m. This salamander is one of many herpetofaunal species endemic to the Cordillera Nombre de Dios. ' © Josiah H. Townsend 909 www.mesoamericanherpetology.com www.eaglemountainpublishing.com Amphibians of the Cordillera Nombre de Dios, Honduras: COI barcoding suggests underestimated taxonomic richness in a threatened endemic fauna JOSIAH H. TOWNSEND1 AND LARRY DAVID WILSON2 1Department of Biology, Indiana University of Pennsylvania, Indiana, Pennsylvania 15705–1081, United States. E-mail: [email protected] (Corresponding author) 2Centro Zamorano de Biodiversidad, Escuela Agrícola Panamericana Zamorano, Departamento de Francisco Morazán, Honduras; 16010 SW 207th Avenue, Miami, Florida 33187-1067, United States. E-mail: [email protected] ABSTRACT: The Cordillera Nombre de Dios is a chain of mountains along the northern coast of Honduras that harbors a high degree of herpetofaunal endemism. We present a preliminary barcode reference library of amphibians from the Cordillera Nombre de Dios, based on sampling at 10 sites from 2008 to 2013. We sequenced 187 samples of 21 nominal taxa for the barcoding locus cytochrome oxidase subunit I (COI), and recovered 28 well-differentiated clades. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -
For Review Only
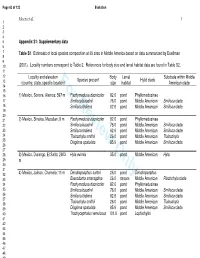
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Froglog95 New Version Draft1.Indd
March 2011 Vol. 95 FrogLogwww.amphibians.org News from the herpetological community The new face of the ASG “Lost” Frogs Red List The global search Updating South comes to an end. Africas Red Where next? Lists. Page 1 FrogLog Vol. 95 | March 2011 | 1 2 | FrogLog Vol. 95 | March 2011 CONTENTS The Sierra Caral of Guatemala a refuge for endemic amphibians page 5 The Search for “Lost” Frogs page 12 Recent diversifi cation in old habitats: Molecules and morphology in the endangered frog, Craugastor uno page 17 Updating the IUCN Red List status of South African amphibians 6 Amphibians on the IUCN Red List: Developments and changes since the Global Amphibian Assessment 7 The forced closure of conservation work on Seychelles Sooglossidae 8 Alien amphibians challenge Darwin’s naturalization hypothesis 9 Is there a decline of amphibian richness in Bellanwila-Attidiya Sanctuary? 10 High prevalence of the amphibian chytrid pathogen in Gabon 11 Breeding-site selection by red-belly toads, Melanophryniscus stelzneri (Anura: Bufonidae), in Sierras of Córdoba, Argentina 11 Upcoming meetings 20 | Recent Publications 20 | Internships & Jobs 23 Funding Opportunities 22 | Author Instructions 24 | Current Authors 25 FrogLog Vol. 95 | March 2011 | 3 FrogLog Editorial elcome to the new-look FrogLog. It has been a busy few months Wfor the ASG! We have redesigned the look and feel of FrogLog ASG & EDITORIAL COMMITTEE along with our other media tools to better serve the needs of the ASG community. We hope that FrogLog will become a regular addition to James P. Collins your reading and a platform for sharing research, conservation stories, events, and opportunities. -

A Taxonomic Re-Evaluation of the Allium Sanbornii Complex
University of the Pacific Scholarly Commons University of the Pacific Theses and Dissertations Graduate School 1986 A taxonomic re-evaluation of the Allium sanbornii complex Stella Sue Denison University of the Pacific Follow this and additional works at: https://scholarlycommons.pacific.edu/uop_etds Part of the Biology Commons Recommended Citation Denison, Stella Sue. (1986). A taxonomic re-evaluation of the Allium sanbornii complex. University of the Pacific, Thesis. https://scholarlycommons.pacific.edu/uop_etds/2124 This Thesis is brought to you for free and open access by the Graduate School at Scholarly Commons. It has been accepted for inclusion in University of the Pacific Theses and Dissertations by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. A TAXONOMIC RE-EVALUATION OF THE ALLIUM SANBORNII COMPLEX A Thesis Presented to the Faculty of the Graduate School University of the Pacific In Partial Fulfillment of the Requirements for the Degree Master of Science by Stella S. Denison August 1986 ACKNOWLEDGMENTS Many contributions have been made for my successful completion of this work. Appreciation is extended to: Drs. Dale McNeal, Alice Hunter, and Anne Funkhouser for their advice and assistance during the research and in the preparation of this manuscript, the entire Biology faculty for their, friendship and suggestions, Ginger Tibbens for the typing of this manuscript, and to my husband, Craig, and my children, Amy, Eric and Deborah for their continued support and encouragement. Grateful acknowledgement is made to the curators of the herbaria from which material was borrowed during this investigation. These herbaria are indicated below by the standard abbreviations of Holmgren and Keuken (1974}. -

Subphyl. Nov., Based on Multi-Gene Genealogies
ISSN (print) 0093-4666 © 2011. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON Volume 115, pp. 353–363 January–March 2011 doi: 10.5248/115.353 Mortierellomycotina subphyl. nov., based on multi-gene genealogies K. Hoffmann1*, K. Voigt1 & P.M. Kirk2 1 Jena Microbial Resource Collection, Institute of Microbiology, University of Jena, Neugasse 25, 07743 Jena, Germany 2 CABI UK Centre, Bakeham Lane, Egham, Surrey TW20 9TY, United Kingdom *Correspondence to: Hoff[email protected] Abstract — TheMucoromycotina unifies two heterogenous orders of the sporangiferous, soil- inhabiting fungi. The Mucorales comprise saprobic, occasionally facultatively mycoparasitic, taxa bearing a columella, whereas the Mortierellales encompass mainly saprobic fungi lacking a columella. Multi-locus phylogenetic analyses based on eight nuclear genes encoding 18S and 28S rRNA, actin, alpha and beta tubulin, translation elongation factor 1alpha, and RNA polymerase II subunits 1 and 2 provide strong support for separation of the Mortierellales from the Mucoromycotina. The existence of a columella is shown to serve as a synapomorphic morphological trait unique to Mucorales, supporting the taxonomic separation of the acolumellate Mortierellales from the columellate Mucoromycotina. Furthermore, irregular hyphal septation and development of subbasally vesiculate sporangiophores bearing single terminal sporangia strongly correlate with the phylogenetic delimitation of Mortierellales, supporting a new subphylum, Mortierellomycotina. Key words — Zygomycetes, SSU rDNA, LSU rDNA, protein-coding genes, monophyly Introduction The type species ofMortierella Coem. 1863, M. polycephala Coem. 1863, was originally isolated from a parasitic interaction with a mushroom and named in honour of M. Du Mortier, the president of the Société de Botanique de Belgique (Coemans 1863). However, the common habit of mortierellalean species is as soil saprobes, enabling the fungi to grow on excrements, decaying plants, or (not infrequently) on decaying mushrooms and mucoralean fungi (Fischer 1892). -

Phylogenetic Inferences in Prunus (Rosaceae) Using Chloroplast Ndhf and Nuclear Ribosomal ITS Sequences 1Jun WEN* 2Scott T
Journal of Systematics and Evolution 46 (3): 322–332 (2008) doi: 10.3724/SP.J.1002.2008.08050 (formerly Acta Phytotaxonomica Sinica) http://www.plantsystematics.com Phylogenetic inferences in Prunus (Rosaceae) using chloroplast ndhF and nuclear ribosomal ITS sequences 1Jun WEN* 2Scott T. BERGGREN 3Chung-Hee LEE 4Stefanie ICKERT-BOND 5Ting-Shuang YI 6Ki-Oug YOO 7Lei XIE 8Joey SHAW 9Dan POTTER 1(Department of Botany, National Museum of Natural History, MRC 166, Smithsonian Institution, Washington, DC 20013-7012, USA) 2(Department of Biology, Colorado State University, Fort Collins, CO 80523, USA) 3(Korean National Arboretum, 51-7 Jikdongni Soheur-eup Pocheon-si Gyeonggi-do, 487-821, Korea) 4(UA Museum of the North and Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK 99775-6960, USA) 5(Key Laboratory of Plant Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China) 6(Division of Life Sciences, Kangwon National University, Chuncheon 200-701, Korea) 7(State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China) 8(Department of Biological and Environmental Sciences, University of Tennessee, Chattanooga, TN 37403-2598, USA) 9(Department of Plant Sciences, MS 2, University of California, Davis, CA 95616, USA) Abstract Sequences of the chloroplast ndhF gene and the nuclear ribosomal ITS regions are employed to recon- struct the phylogeny of Prunus (Rosaceae), and evaluate the classification schemes of this genus. The two data sets are congruent in that the genera Prunus s.l. and Maddenia form a monophyletic group, with Maddenia nested within Prunus. -

Chytridiomycosis Causes Amphibian Mortality Associated with Population Declines in the Rain Forests of Australia and Central America
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 9031–9036, July 1998 Population Biology Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America LEE BERGERa,b,c,RICK SPEAREa,PETER DASZAKd,D.EARL GREENe,ANDREW A. CUNNINGHAMf,C.LOUISE GOGGINg, RON SLOCOMBEh,MARK A. RAGANi,ALEX D. HYATTb,KEITH R. MCDONALDj,HARRY B. HINESk,KAREN R. LIPSl, GERRY MARANTELLIm, AND HELEN PARKESb aSchool of Public Health and Tropical Medicine, James Cook University, Townsville, Queensland 4811, Australia; bAustralian Animal Health Laboratory, Commonwealth Scientific and Industrial Research Organization, Ryrie Street, Geelong, Victoria 3220, Australia; dSchool of Life Sciences, Kingston University, Kingston-upon-Thames, Surrey KT1 2EE, United Kingdom; eMaryland Animal Health Laboratory, College Park, MD 20740; fInstitute of Zoology, Zoological Society of London, Regent’s Park, London NW1 4RY, United Kingdom; gCommonwealth Scientific and Industrial Research Organization, Marine Research, Hobart, Tasmania 7001, Australia; hVeterinary Clinical Centre, University of Melbourne, Werribee, Victoria 3030, Australia; iCanadian Institute for Advanced Research, Program in Evolutionary Biology, National Research Council of Canada, Halifax, NS Canada B3H 3Z1; jConservation Strategy Branch, Queensland Department of Environment, Atherton, Queensland 4883, Australia; kConservation Resource Unit, Queensland Department of Environment, Moggill, Queensland 4070, Australia; lDepartment of Zoology, Southern Illinois University, Carbondale, IL 62901-6501; and mAmphibian Research Centre, 15 Suvla Grove, Nth Coburg, Victoria 3058, Australia Edited by Robert May, University of Oxford, Oxford, United Kingdom, and approved May 18, 1998 (received for review March 9, 1998) ABSTRACT Epidermal changes caused by a chytridiomy- primary degraders or saprobes, using substrates such as chitin, cete fungus (Chytridiomycota; Chytridiales) were found in plant detritus, and keratin. -

02186714 Colombia Finalrepo
2 TABLE OF CONTENTS Project partners & Collaborators ...................................................................................................................................... 3 SECTION 1 ....................................................................................................................................................................... 4 Summary ............................................................................................................................................................................... 4 Introduction ......................................................................................................................................................................... 4 Project members ................................................................................................................................................................. 6 SECTION 2 .....................................................................................................................................................................10 Aim and objectives ......................................................................................................................................................10 Changes to original project plan ...............................................................................................................................10 Methodology ................................................................................................................................................................11