BORAX - Summary of Health Risks Associated with Using Borax in Artisanal and Small-Scale Gold Mining
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Living up to Life Special Stain Kit Modified Grocott's Methenamine
Living up to Life Special Stain Kit Modified Grocott’s Methenamine Silver Stain Catalog No: 38016SS12 Intended Use 16. Rinse slides briefly in deionized water. For In Vitro Diagnostic Use. For Laboratory Use. 17. Dehydrate slides in three changes of absolute alcohol. The reagents in this kit are intended for “In Vitro“ use only. The Modified Grocott’s Methenamine 18. Clear slides in two changes of xylene and mount in a xylene miscible medium. Silver Stain when used with appropriate histological protocols may be used for the demonstration of defined fungi and infectious agents such as Aspergillus sp., Pneumocystis Staining Protocol (Microwave) carinii and Cryptococcus neoformans in formalin fixed, paraffin embedded tissue sections. Exercise caution when using the microwave oven to heat any solution or reagent. The microwave must be properly ventilated to prevent the accumulation of fumes in the laboratory. Probable Mode of Action Microwave transparent Coplin jars and caps should be used during the staining process. The The mechanism of action of Modified Grocott’s Methenamine Silver Stain is based upon the caps should be loosely attached to prevent spills. Caps with ventilation holes also may be used. capacity of aldehyde groups to reduce cationic silver (Ag+) to metallic silver. Chromic acid is All microwave ovens should be used in accordance with the manufacturer’s instructions. The used to generate aldehyde groups by the oxidation of 1-2 glycol groups within polysaccharide procedures described here were performed using an Energy Beam Sciences H2250 laboratory rich tissue components, e.g. glycogen, mucin, reticulin and fungal cell walls. When cationic microwave. -

Scientific Committee on Consumer Safety SCCS
SCCS/1249/09 Revision of 28 September 2010 Scientific Committee on Consumer Safety SCCS OPINION ON Boron compounds The SCCS adopted this opinion at its 7th plenary meeting of 22 June 2010 SCCS/1249/09 Opinion on boron compounds ___________________________________________________________________________________________ About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Jürgen Angerer, Ulrike Bernauer, Claire Chambers, Qasim Chaudhry, Gisela Degen, Gerhard Eisenbrand, Thomas Platzek, Suresh Chandra Rastogi, Vera Rogiers, Christophe Rousselle, Tore Sanner, Kai Savolainen, Jacqueline Van Engelen, Maria Pilar Vinardell, Rosemary Waring, Ian R. -
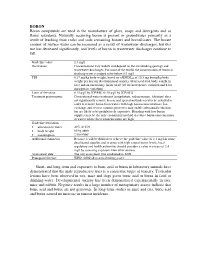
BORON Boron Compounds Are Used in the Manufacture of Glass, Soaps and Detergents and As Flame Retardants
BORON Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall. Guideline value 2.4 mg/l Occurrence Concentrations vary widely and depend on the surrounding geology and wastewater discharges. For most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l. TDI 0.17 mg/kg body weight, based on a BMDL 05 of 10.3 mg boron/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) Limit of detection 0.15 µg/l by ICP/MS; 6–10 µg/l by ICP/AES Treatment performance Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be installed in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. Guideline derivation • allocation to water 40% of TDI • body weight 60 kg adult • consumption 2 litres/day Additional comments Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. -

Synthesis, Characterization and Fungicidal Effects of Some
Journal of Manmohan Memorial Institute of Health Sciences (JMMIHS) Synthesis, Characterization and Fungicidal Effects of some Organoborates Shaheen Akther1 Khanal DP2 Ullah SS3 Abstract Synthesis of benzoyl borate, phthasloyl borate and oxyl borate was done and characterized by infra red, ultraviolet and mass spectra. All newly synthesized and characterized organoborates were tested for their antifungal activity against pencillium that spoilt mostly celluloid materials stored in museum. All synthesized organoborates are unstable in room temperature and quickly degraded on exposure to air. Benzoyl borate found to be most active antifungal compound of the series against penicillium. The fungicidal activity increases in hydrophobicity and electron withdrawing nature of the benzene ring substituents. These compounds may be good fungicidal for the conservation of celluloid materials lying in the museum. Key words: Organoborates, Silver metaborate, Acyl chloride, spore, Antifungal. Introduction All organoborates are synthesized with the used of silver metaborate (AgBO2) and boric acid [1] with organic halides such as 3,5, dinitrobenzoyl chloride and p-nitrobenzoyl chloride. Since the silver atom in AgBO2 in photo activated state the reaction with organic halides having a polarized halogen atom will readily takes place with the formation of silver halides. Steric effect is an important factor in determining the course of reactions well as the stability of the product. It is observed that acyl chloride reacts more readily than sterically hindered pyrollium chloride [2]. The reaction of silver metaborate with organic halides may be ascribed to an ionic mechanism. Indeed acyllium [3] ion have been recognized as possible intermediate in acid catalyzed etherification in Friedel- Crafts reactions through the universality of such mechanism in doubt and the ion is assumed to resonate between the below structures: R – C O+ R – C+ O The presence of CO group polarizes the carbon halogen bond to great extent. -

The Effects of Borax on Milk Yield and Selected Metabolic Parameters in Austrian Simmental (Fleckvieh) Cows
Veterinarni Medicina, 60, 2015 (4): 175–180 Original Paper doi: 10.17221/8104-VETMED The effects of borax on milk yield and selected metabolic parameters in Austrian Simmental (Fleckvieh) cows M. Kabu, C. Uyarlar Faculty of Veterinary Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey ABSTRACT: This study was conducted to determine the effects of orally administered borax on milk yield and on several blood variables related to metabolism in early lactation in Austrian Simmental cows (Fleckvieh). Twenty primiparous cows were selected at parturition and then assigned to one of two groups, the control group or the borax group. The study lasted for four weeks. Borax was administered orally at 0.2 mg/kg/day (Boron group) to all treatment cows shortly after the noon milking, whereas cows in the control group were not treated. All cows consumed the same diet. All feeds in the diet were analysed for crude cellulose, protein, ether extract, ash, and dry matter according to the Weende Analysis Systems, in addition to ADF and NDF, according to Van Soest. Blood samples were collected from all cows via the vena jugularis on lactation Days 0, 7, 15, 21 and 28 and ana- lysed for the following: serum boron (B), non-esterified fatty acids (NEFA), beta-hydroxybutyric acid (BHBA), total cholesterol (TChol), high density lipids (HDL), total protein (TP), blood urea nitrogen (BUN), albumin (ALB), creatine (CRE), uric acid (UA), glucose (GLU), aspartate aminotransferase (AST), alanine aminotrans- ferase (ALT) and gamma-glutamyl transpeptidase (GGT) concentrations. Serum B concentration was higher in the borax group than in the control group at Weeks 1, 2, 3, and 4 of the experiment. -

Available At
Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 VOL. IV.—1890. c THE JOURNAL OF LARYN GO LOGY AND RHINOLOGY; AN ANALYTICAL RECORD OF CURRENT LITERATURE RELATING TO THE THROAT AND NOSE. EDITED BY SIR MORELL MACKENZIE, M.D. Lond., AM) R. NORRIS WOLFENDEN, M.D. Cantab., WITH TUT'. CO-OI'EKAl IuN OF DR. JOHN N. MACKENZIE (BALTIMORE) DR. VALERIUS IDELSON (BERNE) DR. BRYSON DELAVAN (NEW YORK) DR. MICHAEL (HAMBURG) DR. W. PORTER (ST. LOUIS) PROF. RAMON DE LA SOTA Y LASTRA DR. JOAL (MONT DORE) (SEVILLE) PROF. MASSEI (NAPLES) DR. HOLGER MYGIND (COPENHAGEN) PROF. GUYE (AMSTERDAM) DR. SMYLY (DUBLIN) DR. CAPART (BRUSSELS) DR. DAVID COLLINGWOOD (SYDNEY; DR. CONSTANTIN KARWOWSKI (WARSAW) DR. G. W. MAJOR (MONTREAL) DR. BARCLAY J. BARON (BRISTOL) DR. MAXWELL ROSS (EDINBURGH) Dr. G. HUNTER MACKENZIE (Edinburgh). F. A. DAVIS, LONDON: PHILADELPHIA: 40, BERNERS STREET, \V. 1231, FILBERT STREET. Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 CONTENTS. -

The Influence of the Presence of Borax and Nacl on Water
sensors Letter The Influence of the Presence of Borax and NaCl on Water Absorption Pattern during Sturgeon Caviar (Acipenser transmontanus) Storage Massimo Brambilla 1 , Marina Buccheri 2, Maurizio Grassi 2 , Annamaria Stellari 1, Mario Pazzaglia 3, Elio Romano 1 and Tiziana M. P. Cattaneo 2,* 1 Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria–CREA–Centro di Ricerca Ingegneria e Trasformazioni Agroalimentari, Via Milano, 43–24047 Treviglio (Bergamo), Italy; [email protected] (M.B.); [email protected] (A.S.); [email protected] (E.R.) 2 Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria–CREA–Centro di Ricerca Ingegneria e Trasformazioni Agroalimentari, Via G. Venezian, 26–20133 Milano, Italy; [email protected] (M.B.); [email protected] (M.G.) 3 Agroittica Lombarda S.p.A., Via Kennedy, 25012 Calvisano Brescia, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-02-239-557-212 Received: 10 November 2020; Accepted: 10 December 2020; Published: 15 December 2020 Abstract: Sturgeon caviar quality relies not only on the perfect dosage of the ingredients but also on the long sturgeon breeding cycle (about 12–15 years) and the exact timing of the egg extraction. For the improvement and the promotion of Italian caviar, the development of an analytical system dedicated to fish products, and caviar, in particular, is fundamental. The use of near-infrared spectrometry (NIRS) technology is auspicious. The aquaphotomics approach proved to be an adequate analytical tool to highlight, in real-time, the differences in caviar quality stored with, or without, borax as a preservative. -

Safety Data Sheet
Conforms to Regulation (EC) No. 1907/2006 (REACH), Annex II, as amended by Commission Regulation (EU) 2015/830 - Europe SAFETY DATA SHEET SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Product name : Borax decahydrate - Refining Grade Chemical name : Disodium tetraborate decahydrate Index number : 005-011-01-1 EC number : 215-540-4 REACH Registration number Registration number Legal entity 01-2119490790-32-0019 Rio Tinto Iron & Titanium GmbH (5) CAS number : 1303-96-4 Product type : Solid. Other means of : Borax decahydrate, Sodium tetraborate decahydrate, Borax 10 mol identification 1.2 Relevant identified uses of the substance or mixture and uses advised against Material uses : Refer to the table "Identified uses" below. Identified uses Binding agent Chemical production Complexing agent Corrosion inhibitors and anti-scaling agents Fertilisers Flame retardants Flux agents for casting Intermediate Laboratory chemicals Lubricants and lubricant additives Oxidising agents Photosensitive agents and other photo-chemicals pH-regulating agents Plating agents and metal surface treating agents Process regulator (other than polymerisation or vulcanization processes) Process regulator (used in polymerisation or vulcanization processes) Processing aid not otherwise listed Stabilisers Surface active agents Viscosity modifiers A complete list of uses is provided in the introduction to Annex - Exposure Scenarios Uses advised against Reason Consumer uses above the specific concentration limit. Annex XVII - Restrictions on the manufacture, placing on the market and use of certain dangerous substances, mixtures and articles Date of issue/Date of revision : 18/07/2018 Version : 1 1/16 Conforms to Regulation (EC) No. 1907/2006 (REACH), Annex II, as amended by Commission Regulation (EU) 2015/830 Borax decahydrate - Refining Grade SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.3 Details of the supplier of the safety data sheet Borax Europe Limited 6 St. -

Richmond City Health District
In the Bathroom In the Laundry Mold and mildew cleaners Richmond Make a concentrated solution of borax and water Fabric softener City Health and clean affected areas. Use 1/4 to 1/2 cup baking soda in rinse water. Healthy Homes Scrub mildew spots with baking soda or borax. Add 1 cup white vinegar or 1/4 cup baking soda District Sponge with white vinegar. during final rinse. Initiative Scrub with white vinegar and salt. Use 1/2 cup borax and half the amount of detergent per wash load Mix 2 parts baking soda to 1 part borax. Sprinkle Laundry bleach on surfaces, dampen and allow to sit for a time to Use 1/4 cup white vinegar in rinse water to brighten absorb odors. Scrub and dark clothing. Keep It Clean —Safe r rinse. Substitute 1/2 cup borax per wash load to whiten Dissolve 1/2 cup vinegar and whites and brighten colors and to remove spots. Household Cleaners 1/2 cup borax in warm wa- Laundry presoak ter. Soak heavily soiled items in warm water with 1/2 cup washing soda for 30 minutes. To clean mildew from a Rub soiled areas with mild liquid soap. shower curtain, machine wash with 1/4 cup soap Stain Pretreatment and 1/2 cup baking soda; add 1 cup of white vine- Make a paste of baking soda sand water and spread gar to the rinse cycle. on stain. Wet with white vinegar. Soak in soapy water Scouring powder (Kitchen use also) Spray starch Pour 1/2 cup of baking soda into a bowl, add For regular fabrics, dissolve 2 tablespoons cornstarch enough liquid detergent to make a texture-like in a pint of cold water; store in a spray bottle. -

Questions and Answers on Boric Acid and Borates Used As Excipients in Medicinal Products for Human Use
9 October 2017 EMA/CHMP/619104/2013 Committee for Human Medicinal Products (CHMP) Questions and answers on boric acid and borates used as excipients in medicinal products for human use Draft agreed by Excipients Drafting group 16 June 2015 Adopted by CHMP for release for consultation 23 July 2015 Start of public consultation 31 July 2015 End of consultation (deadline for comments) 31 October 2015 Agreed by Excipients Drafting group 16 June 2016 Adopted by CHMP 21 July 2016 Date of publication 9 October 2017 Keywords Excipients, Package leaflet, Boric acid, Borates, Boron, Borax This document should be read in the context of the revision of the Annex of the European Commission guideline ‘Excipients in the labelling and package leaflet of medicinal products for human use’ (EMA/CHMP/3026202017) [1]. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2017. Reproduction is authorised provided the source is acknowledged. Questions and answers on boric acid and borates used as excipients in medicinal products for human use Table of contents Table of contents ......................................................................................... 2 1. What is boric acid and why is it used as an excipient?............................. 3 2. Which medicinal products contain boric acid? ......................................... 3 3. What are the safety concerns? ................................................................ 3 4. What are the reasons for updating the information in the package leaflet? ........................................................................................................ 4 5. Proposal for new information in the package leaflet ............................... 5 Annex - Doses of boric acid, borax and borax anhydrous corresponding to the thresholds determined for boron .......................................................... -

3,032,391 Sttes ICC Patented May 1, 1962
3,032,391 Sttes ICC Patented May 1, 1962 1 2 hydroxide may thus be replaced by the calcium salt of 3,032,391 an organic or inorganic acid. PRODUCTION 0F CALCIUM BORATES According to the present invention, crystalline calcium Geottrey Robert Ball, Belvedere, and Godfrey Harold metaborate (CaB2O4) in the form of its tetrahydrate or Bowden, Epsom, England, assignors to United States hexahydrate is produced by reacting together borax, an Borax & Chemical Corporation, Los Angeles, Cah?, alkali-metal hydroxide (e.g. sodium hydroxide), calcium a corporation of Nevada hydroxide or calcium oxide, and a calcium salt (cg. No Drawing. Filed July 28, 1959, Ser. No. 829,956 Claims priority, application Great Britain Aug. 20, 1958 calcium sulphate or calcium chloride) or mixture of cal 2 Claims. (Cl. 23-59) cium salts, in an aqueous medium, and removing the 10 crystals of hydrated calcium metaborate so obtained. This invention relates to the production of crystalline The Word “borax” is used in this speci?cation to denote calcium borates, and more particularly to the production any sodium 1:2-borate, irrespectively of the number of of crystalline calcium 1,1-borate tetrahydrate and hexa molecules of water of crystallisation (if any). hydrate. Reaction is suitably effected at a temperature of 0° C. There is a large potential demand for pure calcium 15 to 100° C., and preferably at a temperature of 25° C. borate hydrates by the glass industry, and also they to 100° C. The pH value of the aqueous medium is are of great interest to the ceramic industry and in steel preferably 11 or higher. -

Borax,Silver Nitrate-Converted
BORAX ▪ Chemical formula Na2B4O7. 10 H2O ➢ Synonym:- Sodium Borate ➢ Properties:- ➢Colour:-Colourless transparent crystals ➢Odour:-odourless, ➢Taste:- saline and alkaline taste ➢Solubility:- It is soluble in water and glycerin but insoluble in alcohol. ➢Chemical Properties:- ➢On heating loses all its water of hydration & swells to a white porous powder. ➢ When heated to red hot & on cooling , forms a transparent mass known as borax bead 50 ➢ An aqueous sol of Na borate is alkaline to litmus . ➢Alkali borate are water soluble. ➢Borates of other metals undergo hydrolysis & forms insoluble , hydroxides or oxides. Action & Uses:- It has been used externally in solutions containing 1 to 2% of borax as an eyewash, gargle in mouthwashes and as a wet dressing used in food preservative ➢ cosmetics, it is used as emulsifier & also in lotions. germicide & bacteriostatic. Protein precipitantAntimicrobial SILVER NITRATE Chemical formula:-AgNO3 Mol wt : 169.89 preparation:- ➢It is prepared by the action of hot nearly concentrated nitric acid on pure silver. ➢ 3 parts of silver are added to a sol 25% nitric acid ( about 10 parts) heated to expel the nitrous fumes,filltered & evaporated until it is dry. ➢then the mass is fused to convert the copper nitrate into insoluble oxide. So obtained product is dissolved in hot water, filtered it & kept aside in dark place for crystallization. ➢ crystals are collected on sintered glass crucible & dried at 50°c in dark. ➢Purified of AgNO3 is costly therefore pure silver & nitric acid free from halogen & sulphur is used. 63 Properties ➢Colour:-White ➢ Odour:- odourless ➢ Nature:-Rhombic crystals ➢ Taste:- Bitter taste ➢ Solubility:-Very soluble in water ➢Sparingly soluble in alcohol, freely soluble in boiling alcohol It becomes grey/greyish black .