Questions and Answers on Boric Acid and Borates Used As Excipients in Medicinal Products for Human Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Introduction to Boron: History, Sources, Uses, and Chemistry William G
CORE Metadata, citation and similar papers at core.ac.uk Provided by PubMed Central An Introduction to Boron: History, Sources, Uses, and Chemistry William G. Woods Office of Environmental Health and Safety, University of California, Riverside, California Following a brief overview of the terrestrial distribution of boron in rocks, soil, and water, the history of the discovery, early utilization, and geologic origin of borate minerals is summmarized. Modern uses of borate-mineral concentrates, borax, boric acid, and other refined products include glass, fiberglass, washing products, alloys and metals, fertilizers, wood treatments, insecticides, and microbiocides. The chemistry of boron is reviewed from the point of view of its possible health effects. It is concluded that boron probably is complexed with hydroxylated species in biologic systems, and that inhibition and stimulation of enzyme and coenzymes are pivotal in its mode of action. - Environ Health Perspect 102(Suppl 7): 5-11 (1994) Key words: boron, borax, colemanite, glass, boric acid, health effects, enzyme, review Introduction retardants, as micronutrients in fertilizers were an important source of boric acid in Boron is an ubiquitous element in rocks, and for many other purposes, as well. Europe from about 1820 to the 1950s. soil, and water. Most of the earth's soils The varied chemistry and importance Borax also was made from Italian boric have <10 ppm boron, with high concen- of boron is dominated by the ability of acid in England, France, and Germany. trations found in parts of the western borates to form trigonal as well as tetrahe- The borate industry in Turkey com- United States and in other sites stretching dral bonding patterns and to create com- menced in 1865 with mining of the calci- from the Mediterranean to Kazakhstan. -

Living up to Life Special Stain Kit Modified Grocott's Methenamine
Living up to Life Special Stain Kit Modified Grocott’s Methenamine Silver Stain Catalog No: 38016SS12 Intended Use 16. Rinse slides briefly in deionized water. For In Vitro Diagnostic Use. For Laboratory Use. 17. Dehydrate slides in three changes of absolute alcohol. The reagents in this kit are intended for “In Vitro“ use only. The Modified Grocott’s Methenamine 18. Clear slides in two changes of xylene and mount in a xylene miscible medium. Silver Stain when used with appropriate histological protocols may be used for the demonstration of defined fungi and infectious agents such as Aspergillus sp., Pneumocystis Staining Protocol (Microwave) carinii and Cryptococcus neoformans in formalin fixed, paraffin embedded tissue sections. Exercise caution when using the microwave oven to heat any solution or reagent. The microwave must be properly ventilated to prevent the accumulation of fumes in the laboratory. Probable Mode of Action Microwave transparent Coplin jars and caps should be used during the staining process. The The mechanism of action of Modified Grocott’s Methenamine Silver Stain is based upon the caps should be loosely attached to prevent spills. Caps with ventilation holes also may be used. capacity of aldehyde groups to reduce cationic silver (Ag+) to metallic silver. Chromic acid is All microwave ovens should be used in accordance with the manufacturer’s instructions. The used to generate aldehyde groups by the oxidation of 1-2 glycol groups within polysaccharide procedures described here were performed using an Energy Beam Sciences H2250 laboratory rich tissue components, e.g. glycogen, mucin, reticulin and fungal cell walls. When cationic microwave. -

Ri Wkh% Lrorjlfdo (Iihfwv Ri 6Hohfwhg &Rqvwlwxhqwv
Guidelines for Interpretation of the Biological Effects of Selected Constituents in Biota, Water, and Sediment November 1998 NIATIONAL RRIGATION WQATER UALITY P ROGRAM INFORMATION REPORT No. 3 United States Department of the Interior Bureau of Reclamation Fish and Wildlife Service Geological Survey Bureau of Indian Affairs 8QLWHG6WDWHV'HSDUWPHQWRI WKH,QWHULRU 1DWLRQDO,UULJDWLRQ:DWHU 4XDOLW\3URJUDP LQIRUPDWLRQUHSRUWQR *XLGHOLQHVIRU,QWHUSUHWDWLRQ RIWKH%LRORJLFDO(IIHFWVRI 6HOHFWHG&RQVWLWXHQWVLQ %LRWD:DWHUDQG6HGLPHQW 3DUWLFLSDWLQJ$JHQFLHV %XUHDXRI5HFODPDWLRQ 86)LVKDQG:LOGOLIH6HUYLFH 86*HRORJLFDO6XUYH\ %XUHDXRI,QGLDQ$IIDLUV 1RYHPEHU 81,7('67$7(6'(3$570(172)7+(,17(5,25 %58&(%$%%,776HFUHWDU\ $Q\XVHRIILUPWUDGHRUEUDQGQDPHVLQWKLVUHSRUWLVIRU LGHQWLILFDWLRQSXUSRVHVRQO\DQGGRHVQRWFRQVWLWXWHHQGRUVHPHQW E\WKH1DWLRQDO,UULJDWLRQ:DWHU4XDOLW\3URJUDP 7RUHTXHVWFRSLHVRIWKLVUHSRUWRUDGGLWLRQDOLQIRUPDWLRQFRQWDFW 0DQDJHU1,:43 ' %XUHDXRI5HFODPDWLRQ 32%R[ 'HQYHU&2 2UYLVLWWKH1,:43ZHEVLWHDW KWWSZZZXVEUJRYQLZTS Introduction The guidelines, criteria, and other information in The Limitations of This Volume this volume were originally compiled for use by personnel conducting studies for the It is important to note five limitations on the Department of the Interior's National Irrigation material presented here: Water Quality Program (NIWQP). The purpose of these studies is to identify and address (1) Out of the hundreds of substances known irrigation-induced water quality and to affect wetlands and water bodies, this contamination problems associated with any of volume focuses on only nine constituents or the Department's water projects in the Western properties commonly identified during States. When NIWQP scientists submit NIWQP studies in the Western United samples of water, soil, sediment, eggs, or animal States—salinity, DDT, and the trace tissue for chemical analysis, they face a elements arsenic, boron, copper, mercury, challenge in determining the sig-nificance of the molybdenum, selenium, and zinc. -

Carpenter Ants and Control in Homes Page 1 of 6
Carpenter Ants and Control in Homes Page 1 of 6 Carpenter Ants and Control in Homes Fact Sheet No. 31 Revised May 2000 Dr. Jay B Karren, Extension Entomologist Alan H. Roe, Insect Diagnostician Introduction Carpenter ants are members of the insect order Hymenoptera, which includes bees, wasps, sawflies, and other ants. Carpenter ants can be occasional pests in the home and are noted particularly for the damage they can cause when nesting in wood. In Utah they are more of a nuisance rather than a major structural pest. Carpenter ants, along with a number of other ant species, utilize cavities in wood, particularly stumps and logs in decayed condition, as nesting sites. They are most abundant in forests and can be easily found under loose bark of dead trees, stumps, or fallen logs. Homeowners may bring them into their homes when they transport infested logs from forests to use as firewood. Description Carpenter ants include species that are among the largest ants found in the United States. They are social insects with a complex and well-defined caste system. The worker ants are sterile females and may occur in different sizes (majors and minors). Members of the reproductive caste (fertile males and females) are usually winged prior to mating. All ants develop from eggs deposited by a fertilized female (queen). The eggs hatch into grub-like larvae (immatures) which are fed and cared for by the workers. When fully grown, the larvae spin a cocoon and enter the pupal stage. The pupal stage is a period of transformation from the larva to adult. -

Opinion & Information on Boric Acid
Opinion & Information on Boric Acid By Michael R. Cartwright, Sr. (Michael R. Cartwright, Sr. is a third generation licensed professional in the fields of structural pest control and building construction and is also licensed in agriculture pest control. His qualifications are too extensive to print but are available on request from The Reporter.) Over the past years I have seen, in many homes and restaurants, boric acid covering everything. Carpets, floors, toys and furniture, in kitchen cabinets, on counter tops and tables, in refrigerators, clothing, etc. Why? Because environmentalists, helped by an uninformed news media, tell them to. Why don't the news media also explain the possible dangers of applying something not normally found in the home environment, that you or your animals will come in direct contact with? I'm writing this article even though a California environmentalist group advised me not to say anything against boric acid and that I would pay dearly for only trying to mislead the public. My company uses a lot of boric acid, but not as described above. Under an OSHA Hazard Communication Standard, based on animal chronic toxicity studies of inorganic borate chemicals, boric acid and/or borates are Hazardous Materials. California has identified boric acid as a hazardous waste. The above information is taken from Material Safety Data Sheet (MSDS) 25-80-2320 (Section 2 and 13) supplied by U.S. Borax Inc. (the major supplier of borax to many industries). The National Academy of Sciences reports that children may be uniquely sensitive to chemicals and pesticide residues because of their rapid tissue growth and development. -

PLEASE CHECK for LATEST PART INFORMATION 0386006603 Active
This document was generated on 07/30/2021 PLEASE CHECK WWW.MOLEX.COM FOR LATEST PART INFORMATION Part Number: 0386006603 Status: Active Overview: Beau Barrier Strips Description: 6.35mm Pitch Beau PCB Tri-Barrier Terminal Strip, without Mounting Ends, 300V, 3 Circuits Documents: 3D Model 3D Model (PDF) Drawing (PDF) RoHS Certificate of Compliance (PDF) General Series image - Reference only Product Family Terminal Blocks Series 38600 EU ELV Application N/A Not Relevant Component Type One Piece Overview Beau Barrier Strips EU RoHS China RoHS Product Name Fixed Mount Barrier Compliant Type Barrier Strip REACH SVHC UPC 800756242606 Contained Per - D(2020)4578-DC (25 Physical June 2020) Circuits (Loaded) 3 henicosafluoroundecanoic Circuits (maximum) 3 acid Color - Resin Black disodium 4- Entry Angle Horizontal amino-3-[[4'-[(2,4- Lock to Mating Part None diaminophenyl)azo] Material - Metal Brass chromium trioxide Material - Plating Mating Tin 1,3-propanesultone Material - Plating Termination Tin 1-vinylimidazole Material - Resin Polyester Alloy 4,4'-methylenedi-o- Number of Rows 1 toluidine Orientation Horizontal dibutyltin dichloride PC Tail Length 5.10mm methoxyacetic acid PCB Thickness - Recommended 3.18mm 1,2- Panel Mount No Benzenedicarboxylic Pitch - Mating Interface 6.35mm acid, bis(3- Pitch - Termination Interface 6.35mm methylbutyl) Polarized to Mating Part No disodium 3,3'-[[1,1'- Shrouded Tri-Barrier biphenyl]-4,4'- Stackable No diylbis(azo) Surface Mount Compatible (SMC) No octamethylcyclotetrasiloxane Temperature Range - Operating -

Scientific Committee on Consumer Safety SCCS
SCCS/1249/09 Revision of 28 September 2010 Scientific Committee on Consumer Safety SCCS OPINION ON Boron compounds The SCCS adopted this opinion at its 7th plenary meeting of 22 June 2010 SCCS/1249/09 Opinion on boron compounds ___________________________________________________________________________________________ About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Jürgen Angerer, Ulrike Bernauer, Claire Chambers, Qasim Chaudhry, Gisela Degen, Gerhard Eisenbrand, Thomas Platzek, Suresh Chandra Rastogi, Vera Rogiers, Christophe Rousselle, Tore Sanner, Kai Savolainen, Jacqueline Van Engelen, Maria Pilar Vinardell, Rosemary Waring, Ian R. -
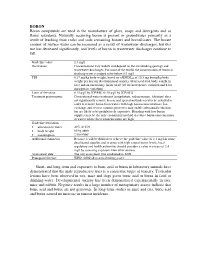
BORON Boron Compounds Are Used in the Manufacture of Glass, Soaps and Detergents and As Flame Retardants
BORON Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall. Guideline value 2.4 mg/l Occurrence Concentrations vary widely and depend on the surrounding geology and wastewater discharges. For most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l. TDI 0.17 mg/kg body weight, based on a BMDL 05 of 10.3 mg boron/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) Limit of detection 0.15 µg/l by ICP/MS; 6–10 µg/l by ICP/AES Treatment performance Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be installed in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. Guideline derivation • allocation to water 40% of TDI • body weight 60 kg adult • consumption 2 litres/day Additional comments Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. -

Synthesis, Characterization and Fungicidal Effects of Some
Journal of Manmohan Memorial Institute of Health Sciences (JMMIHS) Synthesis, Characterization and Fungicidal Effects of some Organoborates Shaheen Akther1 Khanal DP2 Ullah SS3 Abstract Synthesis of benzoyl borate, phthasloyl borate and oxyl borate was done and characterized by infra red, ultraviolet and mass spectra. All newly synthesized and characterized organoborates were tested for their antifungal activity against pencillium that spoilt mostly celluloid materials stored in museum. All synthesized organoborates are unstable in room temperature and quickly degraded on exposure to air. Benzoyl borate found to be most active antifungal compound of the series against penicillium. The fungicidal activity increases in hydrophobicity and electron withdrawing nature of the benzene ring substituents. These compounds may be good fungicidal for the conservation of celluloid materials lying in the museum. Key words: Organoborates, Silver metaborate, Acyl chloride, spore, Antifungal. Introduction All organoborates are synthesized with the used of silver metaborate (AgBO2) and boric acid [1] with organic halides such as 3,5, dinitrobenzoyl chloride and p-nitrobenzoyl chloride. Since the silver atom in AgBO2 in photo activated state the reaction with organic halides having a polarized halogen atom will readily takes place with the formation of silver halides. Steric effect is an important factor in determining the course of reactions well as the stability of the product. It is observed that acyl chloride reacts more readily than sterically hindered pyrollium chloride [2]. The reaction of silver metaborate with organic halides may be ascribed to an ionic mechanism. Indeed acyllium [3] ion have been recognized as possible intermediate in acid catalyzed etherification in Friedel- Crafts reactions through the universality of such mechanism in doubt and the ion is assumed to resonate between the below structures: R – C O+ R – C+ O The presence of CO group polarizes the carbon halogen bond to great extent. -

Transfer of Ingested Insecticides Among Cockroaches: Effects of Active Ingredient, Bait Formulation, and Assay Procedures
HOUSEHOLD AND STRUCTURAL INSECTS Transfer of Ingested Insecticides Among Cockroaches: Effects of Active Ingredient, Bait Formulation, and Assay Procedures 1 2 1, 3 GRZEGORZ BUCZKOWSKI, ROBERT J. KOPANIC, JR., AND COBY SCHAL J. Econ. Entomol. 94(5): 1229Ð1236(2001) ABSTRACT Foraging cockroaches ingest insecticide baits, translocate them, and can cause mor- tality in untreated cockroaches that contact the foragers or ingest their excretions. Translocation of eight ingested baits by adult male Blattella germanica (L.) was examined in relation to the type of the active ingredient, formulation, and foraging area. Ingested boric acid, chlorpyrifos, Þpronil, and hydramethylnon that were excreted by adults in small dishes killed 100% of Þrst instars within 10 d and Ͼ50% of second instars within 14 d. Residues from these ingested baits were also highly effective on nymphs in larger arenas and killed 16Ð100% of the adults. However, when the baits and dead cockroaches were removed from the large arenas and replaced with new cockroaches, only residues of the slow-acting hydramethylnon killed most of the nymphs and adults, whereas residues of fast acting insecticides (chlorpyrifos and Þpronil) killed fewer nymphs and adults. Excretions from cockroaches that ingested abamectin baits failed to cause signiÞcant mortality in cockroaches that contacted the residues. These results suggest that hydramethylnon is highly effective in these assays because cockroaches that feed on the bait have ample time to return to their shelter and defecate insecticide-laden feces. The relatively high concentration of hydramethylnon in the bait (2.15%) and its apparent stability in the digestive tract and feces probably contribute to the efÞcacy of hydra- methylnon. -

Views of the Borax Industry, Ca
http://oac.cdlib.org/findaid/ark:/13030/tf0n39n8j3 Online items available Views of the Borax Industry, ca. 1898-ca. 1915 Processed by Katherine Ruiz. The Bancroft Library, University of California, Berkeley Berkeley, California 94720-6000 1997 Views of the Borax Industry, ca. BANC PIC 1905.17174--PIC 1 1898-ca. 1915 Views of the Borax Industry, ca. 1898-ca. 1915 BANC PIC 1905.17174--PIC The Bancroft Library University of California Berkeley, California1997 Finding aid and digital representations of archival materials funded in part by a grant from the National Endowment for the Humanities. Processed and encoded by: California Heritage Digital Image Access Project staff in The Bancroft Library and The Library's Electronic Text Unit Digital images processed by: The Library Photographic Service Finding aid completed: December 1996 © 1997 The Regents of the University of California Collection Summary Collection Title: Views of the Borax Industry, Date: ca. 1898-ca. 1915 Collection Number: BANC PIC 1905.17174--PIC Creator: Pacific Coast Borax Company Extent: 49 photographic prints ; 20 x 25 cm.49 digital objects Repository: The Bancroft Library. University of California, Berkeley. Berkeley, California 94720-6000 Languages Represented: English Access Collection is available for use. Publication Rights Copyright has not been assigned to The Bancroft Library. All requests for permission to publish photographs must be submitted in writing to the Curator of Pictorial Collections. Permission for publication is given on behalf of The Bancroft Library as the owner of the physical items and is not intended to include or imply permission of the copyright holder, which must also be obtained by the reader. -

The Effects of Borax on Milk Yield and Selected Metabolic Parameters in Austrian Simmental (Fleckvieh) Cows
Veterinarni Medicina, 60, 2015 (4): 175–180 Original Paper doi: 10.17221/8104-VETMED The effects of borax on milk yield and selected metabolic parameters in Austrian Simmental (Fleckvieh) cows M. Kabu, C. Uyarlar Faculty of Veterinary Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey ABSTRACT: This study was conducted to determine the effects of orally administered borax on milk yield and on several blood variables related to metabolism in early lactation in Austrian Simmental cows (Fleckvieh). Twenty primiparous cows were selected at parturition and then assigned to one of two groups, the control group or the borax group. The study lasted for four weeks. Borax was administered orally at 0.2 mg/kg/day (Boron group) to all treatment cows shortly after the noon milking, whereas cows in the control group were not treated. All cows consumed the same diet. All feeds in the diet were analysed for crude cellulose, protein, ether extract, ash, and dry matter according to the Weende Analysis Systems, in addition to ADF and NDF, according to Van Soest. Blood samples were collected from all cows via the vena jugularis on lactation Days 0, 7, 15, 21 and 28 and ana- lysed for the following: serum boron (B), non-esterified fatty acids (NEFA), beta-hydroxybutyric acid (BHBA), total cholesterol (TChol), high density lipids (HDL), total protein (TP), blood urea nitrogen (BUN), albumin (ALB), creatine (CRE), uric acid (UA), glucose (GLU), aspartate aminotransferase (AST), alanine aminotrans- ferase (ALT) and gamma-glutamyl transpeptidase (GGT) concentrations. Serum B concentration was higher in the borax group than in the control group at Weeks 1, 2, 3, and 4 of the experiment.