Views of the Borax Industry, Ca
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Introduction to Boron: History, Sources, Uses, and Chemistry William G
CORE Metadata, citation and similar papers at core.ac.uk Provided by PubMed Central An Introduction to Boron: History, Sources, Uses, and Chemistry William G. Woods Office of Environmental Health and Safety, University of California, Riverside, California Following a brief overview of the terrestrial distribution of boron in rocks, soil, and water, the history of the discovery, early utilization, and geologic origin of borate minerals is summmarized. Modern uses of borate-mineral concentrates, borax, boric acid, and other refined products include glass, fiberglass, washing products, alloys and metals, fertilizers, wood treatments, insecticides, and microbiocides. The chemistry of boron is reviewed from the point of view of its possible health effects. It is concluded that boron probably is complexed with hydroxylated species in biologic systems, and that inhibition and stimulation of enzyme and coenzymes are pivotal in its mode of action. - Environ Health Perspect 102(Suppl 7): 5-11 (1994) Key words: boron, borax, colemanite, glass, boric acid, health effects, enzyme, review Introduction retardants, as micronutrients in fertilizers were an important source of boric acid in Boron is an ubiquitous element in rocks, and for many other purposes, as well. Europe from about 1820 to the 1950s. soil, and water. Most of the earth's soils The varied chemistry and importance Borax also was made from Italian boric have <10 ppm boron, with high concen- of boron is dominated by the ability of acid in England, France, and Germany. trations found in parts of the western borates to form trigonal as well as tetrahe- The borate industry in Turkey com- United States and in other sites stretching dral bonding patterns and to create com- menced in 1865 with mining of the calci- from the Mediterranean to Kazakhstan. -

Effect of Borate Addition on the Sintered Properties of Pulverised Fuel Ash E.A
Ceramics International 33 (2007) 993–999 www.elsevier.com/locate/ceramint Effect of borate addition on the sintered properties of pulverised fuel ash E.A. Uwe a, A.R. Boccaccini b, S.G. Cook c, C.R. Cheeseman a,* a Centre for Environmental Control and Waste Management, Department of Civil and Environmental Engineering, Imperial College London, SW7 2BU, UK b Department of Materials, Imperial College London, SW7 2BP, UK c Borax Europe Limited, 1A Guildford Business Park, Guildford GU2 8XG, UK Received 15 December 2005; received in revised form 8 January 2006; accepted 22 February 2006 Available online 25 April 2006 Abstract The effect of borate addition on the properties of sintered pulverised fuel ash (PFA) has been investigated. PFA from a major coal-fired power station in the UK has been formed into monolithic ceramic samples using dry powder compaction and sintering. Borax pentahydrate (Na2B4O7Á5H2O), anhydrous borax (Na2B4O7) and boric acid (H3BO3) were individually added to the PFA and the effects of firing between 1000 and 1200 8C have been investigated. Physical properties (density, water absorption and linear shrinkage), mechanical properties (bending strength and Vickers hardness), phase composition (X-ray diffraction) and sintered microstructure (scanning electron microscopy) are reported. Milling PFA containing 8% (by weight) of borax pentahydrate, to an average particle size of 7.25 mm and sintering at 1130 8C for 1 h, produces ceramics with properties comparable to those of commercial unglazed ceramic wall and floor tiles. The results indicate that PFA could be used to produce commercial ceramics using an economic manufacturing process, although current formulations display excessive shrinkage. -

14 Boron Element Pub 2020
Publication WSFNR-20-14C March 2020 BORON (B) – Tree Essential Element Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources Boron (B) is a hard, brittle, brown, but light weight solid called a metalloid discovered in 1808. Its name is derived from Persian for a boron containing compound. The rest of its elemental family are all metals. It is never found in pure form. It is used for cleaning products, water softening, antiseptics, heat resistant glass, nuclear reactor rods, and transistors. In Trees Boron is a rare element and the lightest (except for hydrogen) element essential for trees. Boron is one of two essential elements (i.e. silicon is the other) usually present in a tree as a neutral molecule (undissociated form) rather than as an ion. The concentration of boron can vary widely in tissues. Its form in a tree depends upon tissue pH, existing as boric acid (B(OH)3 / H3BO3) where pH is less than 7.0 and the borate ion (B(OH)4-) where pH >7.5. Figure 1. At soil pH of 6.5 to 10.0, boron is poorly available or unavailable to trees. Boron performs one dominant role in trees as part of cell wall structure. Boron helps bind pectic polysaccharides in and between tree cell walls. Deficiency symptoms can quickly occur physiologically and structurally. Element Availability Problems Boron deficiency causes many biological and structural problems in trees. Boron can act as an antibiological agent in trees and when it is in short supply pathogens attacks are more effective and move quicker. -

Barry Lawrence Ruderman Antique Maps Inc
Barry Lawrence Ruderman Antique Maps Inc. 7407 La Jolla Boulevard www.raremaps.com (858) 551-8500 La Jolla, CA 92037 [email protected] Wm. T. Coleman & Co.'s Map of the State of California [and] Wm. T Coleman& Co's. Skeleton Map of Columbia River Canneries, Showing Their Location. Stock#: 69974 Map Maker: Coleman Date: 1885 Place: n.p. Color: Color Condition: VG+ Size: 9 x 12.5 inches Price: SOLD Description: Exceptional Chromolithographic Promotional Maps Rare promotional maps of California the Columbia River Basin one verso, promoting the shipping business of pioneer California Businessman William Tell Coleman. The California map locates several domestic industries, including Borax, Salmon, Orange, Fruit & Raisin, and Wine & Brandy Districts. On the verso, the map shows a list of 24 Canneries operating on the Columbia River near Astoria, with about another 10 also located on the map. Each side is embellished with a striking graphic, Yosemite on the California side, and Mount Hood on the Columbia River side. Drawer Ref: Small Maps Stock#: 69974 Page 1 of 2 Barry Lawrence Ruderman Antique Maps Inc. 7407 La Jolla Boulevard www.raremaps.com (858) 551-8500 La Jolla, CA 92037 [email protected] Wm. T. Coleman & Co.'s Map of the State of California [and] Wm. T Coleman& Co's. Skeleton Map of Columbia River Canneries, Showing Their Location. William Tell Coleman William Tell Coleman (1824–1893) was an American pioneer in California. Coleman came to California in 1849 and settled in San Francisco, where he engaged in the shipping and commission business. Coleman was a leading figure in both the 1851 and 1856 Committees of Vigilance, which usurped civic power to drive out the Democratic Party machine and ostensibly establish law and order. -

THE EFFECT of BORATE on the OXYGEN UPTAKE of BRAIN TISSUE in KREBS PHOSPHATE RINGER SOLUTION I. INTRODUCTION Cases of Borate
THE EFFECT OF BORATE ON THE OXYGEN UPTAKE OF BRAIN TISSUE IN KREBS PHOSPHATE RINGER SOLUTION By E. M. TRAUTNER~ and M. MESSER~ [Manuscript received February 26, 1953] Summary The effect of borate on the respiration of guinea pig brain cells in Krebs phosphate Ringer solution in the absence of any added substrate and in the pre sence of glucose and fructose has been investigated. In the absence of substrate borate rapidly depresses the oxygen uptake, causing finally a very low, apparently constant "borate-resistant" respiration. In the presence of glucose in concentrations sufficient to maintain the respi ration rate of the tissue in the absence of borate, the addition of borate in 50 to 100 times excess over the glucose has no early effect on the respiration. To inhibit the effects of borate to the same degree, fructose has to be pre sent in four to six times higher concentration than glucose. In the absence of additional hexose, the R.Q. of the tissue respiring in the presence of borate falls rapidly below 0.9. In the presence of a sufficient con centration of the two sugars it remains unaltered, slightly lower than 1.0. Addition of 0.03M mannitol to the medium has no effect on the action of borate. Glucose in very low concentrations maintains the normal respiration rate of the tissue in the presence of borate for a period depending on its concentration. Increasing concentrations progressively delay the onset of the respiratory depres sion. Fructose in low concentrations fails to maintain the respiratory rate; in creasing concentrations merely raise the oxygen uptake until it coincides with that of the borate-free control. -

Process for Regeneration of Sodium Borate to Sodium Borohydride for Use As a Hydrogen Storage Source, Excerpt from DOE Hydrogen
IV.B CheMicaL SToraGE IV.B.1 Process for Regeneration of Sodium Borate to Sodium Borohydride for Use as a Hydrogen Storage Source (K) System Life-Cycle Assessments Ying Wu (Primary Contact), Michael T. Kelly, (R) Regeneration Processes Jeffrey V. Ortega, and Oscar A. Moreno (S) By-Product/Spent Material Removal Millennium Cell Inc. 1 Industrial Way West, Building E Eatontown, NJ 07724 Technical Targets Phone: (732) 544-5718; Fax: (732) 542-2846 E-mail: [email protected] Current manufacturing practice and the accepted market price of NaBH4 give rise to an equivalent DOE Technology Development Manager: hydrogen cost in the range of $188-259/kg H2. Grace Ordaz Regeneration and spent material management are key Phone: (202) 586-8350; Fax: (202) 586-9811 aspects of the electrochemical syntheses. Efficiency E-mail: [email protected] is being evaluated through reaction engineering assessment. Several interim cost targets have been set, DOE Project Officer: Jim Alkire leading to the final target of meeting DOE’s $2-3/kg 2H Phone: (303) 275-4795; Fax: (303) 275-4753 goal. E-mail: [email protected] • Interim Target 1: Improved Na Electrolysis Yields Contract Number: DE-FC36-04GO14008 $50/kg H2 via $10/kg NaBH4 Subcontractors: • Interim Target 2: Incorporation of Waste Material Air Products and Chemicals Inc., Allentown, PA Recycling: $2/kg NaBH4 and $10/kg H2 Princeton University, Princeton, NJ • Interim Target 3: One-Pot Electrochemical Method: $1/kg NaBH and $5/kg H Start Date: November 1, 2003 4 2 Projected End Date: September 30, 2007 Accomplishments • Demonstrated the feasibility of reducing NaBH4 Objectives manufacturing cost and projected cost reduction by a factor of three through a more efficient and • The primary objective is to develop low-cost less costly Na production process. -

Material Safety Data Sheet Sodium Borate, Decahydrate Section 1
ISO9001:2000 Certified Material Safety Data Sheet Sodium Borate, Decahydrate Section 1 - Chemical Product and Company Identification MSDS Name: Sodium borate, decahydrate Catalog Numbers: LC22955 Synonyms: Borax; Sodium tetraborate decahydrate; Disodium tetraborate decahydrate. Company Identification: LabChem Inc 200 William Pitt Way Pittsburgh, PA 15238 Company Phone Number: (412) 826-5230 Emergency Phone Number: (800) 424-9300 CHEMTREC Phone Number: (800) 424-9300 Section 2 – Composition, Information on Ingredients CAS# Chemical Name: Percent 1303-96-4 Sodium tetraborate, decahydrate 100 Section 3 - Hazards Identification Emergency Overview Appearance: White solid. Danger! Causes eye, skin, and respiratory tract irritation. May impair fertility. May cause harm to the unborn child. Target Organs: None. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. May be harmful if absorbed through the skin. Ingestion: May cause irritation of the digestive tract. Human fatalities have been reported from acute poisoning. Inhalation: May cause respiratory tract irritation. Chronic: Chronic exposure may cause reproductive disorders and teratogenic effects. - 1 - Material Safety Data Sheet Sodium Borate, Decahydrate Section 4 - First Aid Measures Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Ingestion: Call a poison control center. If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid. Inhalation: Remove from exposure and move to fresh air immediately. -

Questions and Answers on Boric Acid and Borates Used As Excipients in Medicinal Products for Human Use
9 October 2017 EMA/CHMP/619104/2013 Committee for Human Medicinal Products (CHMP) Questions and answers on boric acid and borates used as excipients in medicinal products for human use Draft agreed by Excipients Drafting group 16 June 2015 Adopted by CHMP for release for consultation 23 July 2015 Start of public consultation 31 July 2015 End of consultation (deadline for comments) 31 October 2015 Agreed by Excipients Drafting group 16 June 2016 Adopted by CHMP 21 July 2016 Date of publication 9 October 2017 Keywords Excipients, Package leaflet, Boric acid, Borates, Boron, Borax This document should be read in the context of the revision of the Annex of the European Commission guideline ‘Excipients in the labelling and package leaflet of medicinal products for human use’ (EMA/CHMP/3026202017) [1]. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2017. Reproduction is authorised provided the source is acknowledged. Questions and answers on boric acid and borates used as excipients in medicinal products for human use Table of contents Table of contents ......................................................................................... 2 1. What is boric acid and why is it used as an excipient?............................. 3 2. Which medicinal products contain boric acid? ......................................... 3 3. What are the safety concerns? ................................................................ 3 4. What are the reasons for updating the information in the package leaflet? ........................................................................................................ 4 5. Proposal for new information in the package leaflet ............................... 5 Annex - Doses of boric acid, borax and borax anhydrous corresponding to the thresholds determined for boron .......................................................... -

Arbor Villa: the Home of Mr. & Mrs. F. M. Smith, Photographed by E. T
http://oac.cdlib.org/findaid/ark:/13030/tf509nb66b Online items available Arbor Villa: the Home of Mr. & Mrs. F. M. Smith, Photographed by E. T. Dooley, 1902 Processed by Padma Rubiales. The Bancroft Library, University of California, Berkeley Berkeley, California 94720-6000 1997 Arbor Villa: the Home of Mr. & BANC PIC 1950.005--ffALB 1 Mrs. F. M. Smith, Photographed by E. T. Dooley, 1902 Arbor Villa: the Home of Mr. & Mrs. F. M. Smith, Photographed by E. T. Dooley, 1902 BANC PIC 1950.005--ffALB The Bancroft Library University of California Berkeley, California1997 Finding aid and digital representations of archival materials funded in part by a grant from the National Endowment for the Humanities. Processed and encoded by: California Heritage Digital Image Access Project staff in The Bancroft Library and The Library's Electronic Text Unit Digital images processed by: The Library Photographic Service Finding aid completed: October 1996 © 1997 The Regents of the University of California Collection Summary Collection Title: Arbor Villa: the Home of Mr. & Mrs. F. M. Smith, Photographed by E. T. Dooley Collection Number: BANC PIC 1950.005--ffALB Extent: 47 photoengravings in 1 album; 36 x 50 cm.47 digital objects Photographer: E. T. Dooley Repository: The Bancroft Library. University of California, Berkeley. Berkeley, California 94720-6000 Languages Represented: English Access Collection stored off-site; advance notice required for use. Publication Rights Copyright has not been assigned to The Bancroft Library. All requests for permission to publish photographs must be submitted in writing to the Curator of Pictorial Collections. Permission for publication is given on behalf of The Bancroft Library as the owner of the physical items and is not intended to include or imply permission of the copyright holder, which must also be obtained by the reader. -

I798 JACKSON Jackson, Phil, 1349 Jackson, Thomas Penfield, Ro3 8
I798 JACKSON INDEX INDEX JORDAN MCGRATH CASE & TAYLOR, INC. I799 798, I2oI, I266, I52I, i601; Thrille~ Japan Advertising Agency Association, 466, Jennings, Peter, 95, 523 Joe Camel, 311, 316, 336, 539, 690, 760, Johnson, Ray, 77 (Durham, North Carolina). See under r520; "Thrille1;" Io92 469, 878 Jenny Craig International. See under Craig 1212, 1369, 1694 Johnson, Robert Wood, Jr., 880, 881, 882 Harttnan Jackson, Phil, 1349 Japan Advertising Council, 468 Jeno's Pizza, 623, 1234, 1235 Joe Chemo, 336 Johnson, Robert Wood, Sr., 880 John W. Shaw Advertising. See under Shaw Jackson, Reggie, 148r, I482 Japan Air Lines, 898, 900, 1462 Jensen, Thomas, 525 Joe Isuzu, 114, II4, 457, 475, 522 Johnson, Samuel, 749 John Wanamaker & Company. See under Jackson, Thomas Penfield, ro3 8 Japan Audit Bureau of Circulation Jenson, Nicolas, 1580, 1583 foe Palooka, 729 Johnson, Samuel Curtis, 884, 886, 888 Wanamaker Jackson & Perkins, 1673 Association, 466 Jenson typefaces, 1580, 1583, 1584 Joe Robbie Stadiun1, 335 Johnson, William H., 139 "Join the Dodge rebellion," 305 Jacksonville Jaguars, 3 3 5 japan Marketing Association, 466 Jeopardy!, 1426. See also color plate in "Jogger," 940 Johnson, William R., 730 "Join the people who've joined the Army," Jackson Wain Agency, 1071 144, 238, I437 Japan Newspaper Advertising Agency volume one John, W.A.P., 791 Mead Johnson & Co1npany, 57, 210, 880 1050 Jack Tinker & Partners. See under Tinker Association, 466 Jep et Carre, 618 "John and Marsha" (Freberg), 623, 824 Johnson & Johnson, 582, 880-83; Ammirati Joint Policy Com1nittee for Broadcast Talent Jacob Ruppert, Inc. See under Ruppert Jardine Matheson & Company, 301 ]e Reviens, 1202 John Birch Society, 1676 Puris Lintas and, 967; Batten Barton Union Relations, 99 Jacobs, Alan, 197 Jarreau, Al, 1395 Andrew Jergens Company: Cunningham & John Brown & Partners. -

View March 2017
2 —————————— The Peconic Bay Shopper • Preserving Local History • MARCH 2017 —————————— Taylor’s Island publisher/editor — Michael P. Hagerman art department — Rita M. Hagerman | [email protected] advertising sales — Kristin Ulmet 631.466.8363 | [email protected] This publication is a division of Academy Printing Services, Inc. 42 Horton Lane - POB 848, Southold NY 11971 PH 631.765.3346 FAX 631.765.3369 EMAIL [email protected] Taylor’s Island is a tombolo in Coecles Harbor, Shelter Island. The Island and its historic Smith-Taylor Cabin, built around 1900 by F. The Peconic Bay Shopper is published monthly, excluding January. M. Smith. Taylor’s Island (deed) was left to the Town of Shelter Island Recent issues can be viewed and downloaded at “for the use and enjoyment of the general public” by S. Gregory Taylor www.academyprintingservices.com (Soterios Gregorios Tavoulares) in his last will and testament and ac- cepted by the Town of Shelter Island in 1979. Under the terms of the will, Mr. Taylor’s nephew, Stephen Stephano, had the use of Taylor’s Island until his death, which occurred in 1997. The Town took actual possession of the Island in 1998. The Taylor’s Island Preservation and Management Committee and the Taylor’s Island Foundation exist to restore the Island and fulfill Mr. Taylor’s wish. Francis Marion Smith, of 20-Mule Team Borax fame, on the porch of the The Smith-Taylor Cabin is listed on the New York State Register of Adirondack style Cabin he built on Cedar Island, Coecles Harbor with his wife Historic Places and on the National Register of Historic Places. -
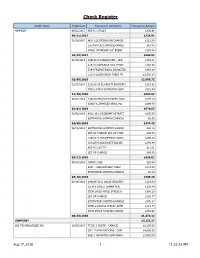
Check Register
Check Register Vendor Name Check Date Transaction Description Transaction Amount 4IMPRINT 09/21/2017 REF PO 175823 $326.91 09/21/2017 $326.91 10/19/2017 ADD' LOCATION RUN CHARGE $121.50 ESTIMATED SHIPPING/HANDLI $47.82 #8921 STANDARD 10" EVENT $391.50 10/19/2017 $560.82 02/08/2018 109148-S CUBANO PEN - OPA $348.21 119373 ADHESIVE CELL PHON $192.98 7194 PROMOTIONAL DRAWSTRI $489.19 2213 CLOSED BACK TABLE TH $1,050.34 02/08/2018 $2,080.72 02/15/2018 134226-10 ECONOMY BACKDRO $316.88 39212-STS-S STANDARD SHAP $322.94 02/15/2018 $639.82 03/01/2018 144160 RECYCLED PAPER TWO $275.14 85015-S ZIPPERED VINYL PO $699.79 03/01/2018 $974.93 03/08/2018 8922-36-L ECONOMY RETRACT $265.50 ESTIMATED SHIPPING/HANDLI $8.95 03/08/2018 $274.45 06/13/2018 ESTIMATED SHIPPING/HANDLI $40.18 SET-UP CHARGE SET-UP CHAR $49.50 146012-5 TRIUMPHANT ACRYL $296.34 110325-S ASSOCIATE RINGBI $249.49 REF PO 183779 $11.01 SET UP CHARGE $49.50 06/13/2018 $696.02 06/20/2018 CARRY CASE $25.00 5957 - CONVERTIBLE TABLE $174.89 ESTIMATED SHIPPING/HANDLI $8.49 06/20/2018 $208.38 08/16/2018 106836-1312 VALUE GROCERY $129.00 ITEM #102557 SAMBA PEN $250.44 ITEM 18035 APPLE STRESS R $248.28 SET UP CHARGE $209.77 ESTIMATED SHIPPING/HANDLI $165.17 ITEM #108/463 SMILEY ADHE $213.74 ITEM #6622 5-PRONG HIGHLI $256.92 08/16/2018 $1,473.32 4IMPRINT $7,235.37 806 TECHNOLOGIES INC 10/05/2017 TITLE 1 CRATE - CAMPUS $2,250.00 CIP - PLAN4LEARNING - CAM $4,000.00 BULLY REPORTER SOFTWARE - $3,600.00 Aug 17, 2018 - 1 - 11:22:13 AM Check Register Vendor Name Check Date Transaction Description Transaction