MACP200 Chemical Compatability Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Sulfate
FT-08762A Sodium Sulfate Product Information Chemical name : Sodium Sulfate, ACS grade Syn.: Na2SO4 , sodium salt of sulfuric acid, Disodium sulfate; Bisodium sulfate; Dibasic sodium sulfate; Disodium monosulfate; (All.): disodium sulphate; Natriumsulfat; sodium sulphate CAS: 7757-82-6 EC number: 231-820-9 Cat. Number : 08762A, 500g 08762B, 1Kg 08762C, 2.5Kg 08762-B, Bulk Structure : Na2SO4 (anh.) Molecular Weight : 142.04 Properties: Form: white solid crystalline powder (hygroscopic) Density: 2.664 g/cm3 Melting point: 884°C Boiling point: 1429°C Refractive index (nD)/ 1.468 pKa: 10.329 and 6.351(carbonic acid) Solubility: readily soluble in water >4% w/v Storage: Room temperature (Z) Safety: Irritant. Non-flammable. Risk Statements: 36/37/38 Safety Statements: 36/37/39 Applications: Suitable for many biochemistry and biotechnology applications. P.1 FT-08762A Specifications Test Specifications Purity >99.0% Calcium (%) 0.01 Chloride (%) 0.001 Heavy Metals (as Pb) 0.0005 Iron (%) 0.001 Magnesium (%) 0.005% Nitrogen compounds (%) 0.0005% Phosphates (%) 0.001% Insolubles 0.001% Loss on Ignition (%) 0.5 pH (5%, water, 25°C) 0.01 + Technical information ●Chemistry Sodium sulfate is a neutral salt, which forms aqueous solutions with pH of 7. The Na+ ion weakly polarizes its water lig- ands provided there are metal ions in solution. Sodium sulfate reacts with sulfuric acid to give the acid salt sodium bisulfate, leading ot a temperatude-dependant equi- librium: Na2SO4 + H2SO4 ⇌ 2 NaHSO4 2− Sulfate ions (SO4 ) in solution can be indicated by the easy formation of insoluble sulfates when these solutions are treated with Ba2+ or Pb2+ salts: Na2SO4 + BaCl2 → 2 NaCl + BaSO4 Double salts with some other alkali metal sulfates are known, including Na2SO4·3K2SO4. -

Living up to Life Special Stain Kit Modified Grocott's Methenamine
Living up to Life Special Stain Kit Modified Grocott’s Methenamine Silver Stain Catalog No: 38016SS12 Intended Use 16. Rinse slides briefly in deionized water. For In Vitro Diagnostic Use. For Laboratory Use. 17. Dehydrate slides in three changes of absolute alcohol. The reagents in this kit are intended for “In Vitro“ use only. The Modified Grocott’s Methenamine 18. Clear slides in two changes of xylene and mount in a xylene miscible medium. Silver Stain when used with appropriate histological protocols may be used for the demonstration of defined fungi and infectious agents such as Aspergillus sp., Pneumocystis Staining Protocol (Microwave) carinii and Cryptococcus neoformans in formalin fixed, paraffin embedded tissue sections. Exercise caution when using the microwave oven to heat any solution or reagent. The microwave must be properly ventilated to prevent the accumulation of fumes in the laboratory. Probable Mode of Action Microwave transparent Coplin jars and caps should be used during the staining process. The The mechanism of action of Modified Grocott’s Methenamine Silver Stain is based upon the caps should be loosely attached to prevent spills. Caps with ventilation holes also may be used. capacity of aldehyde groups to reduce cationic silver (Ag+) to metallic silver. Chromic acid is All microwave ovens should be used in accordance with the manufacturer’s instructions. The used to generate aldehyde groups by the oxidation of 1-2 glycol groups within polysaccharide procedures described here were performed using an Energy Beam Sciences H2250 laboratory rich tissue components, e.g. glycogen, mucin, reticulin and fungal cell walls. When cationic microwave. -

Sodium Bisulfate
Sodium Bisulfate Livestock 1 Identification of Petitioned Substance 2 Chemical Names: Sodium bisulfate 3 IUPAC1 name: Sodium hydrogen sulfate 4 5 Other Name: 6 Hydrogen sodium sulfate 7 Monosodium hydrogen sulfate 8 Sodium acid sulfate 9 Sulfuric acid sodium salt 10 Bisulfate of soda 11 Niter cake 12 Fanal 13 14 Trade Names: PLT® (Poultry Litter Treatment) 15 CAS Numbers: 7681-38-1 (anhydrous) and 13324-88-5 (monohydrate) Other Codes: Pesticide registration number 33907-3 EINECS2 231-665-7 EPA PC code 073201; 873201 1 International Union of Pure and Applied Chemistry 2 European chemical substances information system ___________________________________ May 8, 2015 Technical Evaluation Report Page 1 of 17 Compiled by OMRI for the USDA National Organic Program Sodium Bisulfate Livestock 16 Summary of Petitioned Use 17 18 The petitioned purpose for sodium bisulfate, in the form of the commercial product PLT® (currently there 19 are no other commercial forms of sodium bisulfate designed to be used as a litter treatment), is to control 20 ammonia in poultry houses for all species of domestic fowl in orders Galliformes (includes chickens, 21 turkeys, quail, pheasant, etc.) and Anseriformes (waterfowl). It is intended as a topical litter and dirt pad 22 treatment. It is not intended for use in feed, food or drinking water. It is being petitioned for addition to 23 §205.603 as a poultry litter additive. According to the petitioner, litter amendments such as sodium 24 bisulfate minimize ammonia volatilization, improving poultry health and maximizing the litter’s 25 agronomic, environmental, and financial value. 26 27 Characterization of Petitioned Substance 28 29 Composition of the Substance: 30 + - 31 Sodium bisulfate is the sodium (Na ) salt of the bisulfate anion (HSO4 ) and has the molecular formula of 32 NaHSO4. -

Scientific Committee on Consumer Safety SCCS
SCCS/1249/09 Revision of 28 September 2010 Scientific Committee on Consumer Safety SCCS OPINION ON Boron compounds The SCCS adopted this opinion at its 7th plenary meeting of 22 June 2010 SCCS/1249/09 Opinion on boron compounds ___________________________________________________________________________________________ About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Jürgen Angerer, Ulrike Bernauer, Claire Chambers, Qasim Chaudhry, Gisela Degen, Gerhard Eisenbrand, Thomas Platzek, Suresh Chandra Rastogi, Vera Rogiers, Christophe Rousselle, Tore Sanner, Kai Savolainen, Jacqueline Van Engelen, Maria Pilar Vinardell, Rosemary Waring, Ian R. -

Sulfur (IV) Isotopic Exchange Reaction in Aqueous and Concentrated Acid
THE KINETICS OF THE SULFtJR(IV) - suLFuR(vI) ISOTOPIC EXCHANGE REACTION IN AQUEOUS AND CONCTRATED ACID )LUTIONS by RAY LOCKE McDONALD A THESIS submitted to OHEGON STATE COLLEGE In parti1 fulfillment of the requirements for the degree of DOCTOR 0F PHW)SOPHY June 196]. flIiY1i$IT Redacted for Privacy Professor of Chemistry In Charge of Major Red acted f or P rivacy Chairman of Department of Cnemistry Redacted for Privacy Chairman of School Graduate Committee Redacted for Privacy Dean of Graduate School nate thesis is presented Typed by LeAnna kiarris tffi*ffimffi Fcar rdsrmo ad mflss. dte rU egestr d lilt rretc Mlr1 tb lutEm'rprm [ilr;r* dffi tldr te EufUe ?. E. I*1ill. TABLE OF CONTENTS Page I. INTRODUCTION ...................... i II. E(PERIMENTAL ...................... 7 A. General Procedure ................. 7 B. Radioactivity Analysis ............... 9 C. Chemical Analysis ................ il D, Preparation of Materials and Reactant Solutions 13 1. General ................. 13 2. Sulfur Dioxide ................ 1.3 3. Labeled Aqueous Sulfuric Acid ......... i1 )4. Labeled Concentrated Sulfuric cid ....... 15 ;. Labeled 100% Sulfuric Acid ........... 16 6. Labeled Fuming Sulfuric Acid .......... 16 7. Labeled Aqueous Sodium Bisulfate ........ 16 8. Lat.ed Sodium Bisulfate in Aqueous Sulfuric Acid .................. 17 9. Labeled Sodium Bisulfate in Concentrated . Sulfuric Acid .................. 17 10. Labeled Sodium Sulfate ............. 17 li. Labeled Sodium Sulfate in Aqueous Sodium Bisulfate ................ 18 12. Labeled Elemental Sulfur ............ 18 III. RUN PROCEDURE AND DATA ................ 19 A. Sulfur(IV) - Sulfur(VI) Exchange in Basic Media . 19 B, - Sulfur(IV) Sulfur(VI) Exchange in Acidic Media . .23 1. Radiosulfur Ecchsuge Experiments Between Sulfur Dioxìe and Aqueous Sulfuric Acid of High Specific Activity ........... -
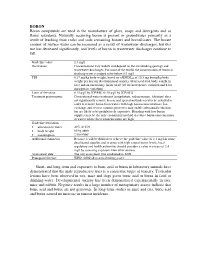
BORON Boron Compounds Are Used in the Manufacture of Glass, Soaps and Detergents and As Flame Retardants
BORON Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall. Guideline value 2.4 mg/l Occurrence Concentrations vary widely and depend on the surrounding geology and wastewater discharges. For most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l. TDI 0.17 mg/kg body weight, based on a BMDL 05 of 10.3 mg boron/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) Limit of detection 0.15 µg/l by ICP/MS; 6–10 µg/l by ICP/AES Treatment performance Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be installed in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. Guideline derivation • allocation to water 40% of TDI • body weight 60 kg adult • consumption 2 litres/day Additional comments Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. -

Synthesis, Characterization and Fungicidal Effects of Some
Journal of Manmohan Memorial Institute of Health Sciences (JMMIHS) Synthesis, Characterization and Fungicidal Effects of some Organoborates Shaheen Akther1 Khanal DP2 Ullah SS3 Abstract Synthesis of benzoyl borate, phthasloyl borate and oxyl borate was done and characterized by infra red, ultraviolet and mass spectra. All newly synthesized and characterized organoborates were tested for their antifungal activity against pencillium that spoilt mostly celluloid materials stored in museum. All synthesized organoborates are unstable in room temperature and quickly degraded on exposure to air. Benzoyl borate found to be most active antifungal compound of the series against penicillium. The fungicidal activity increases in hydrophobicity and electron withdrawing nature of the benzene ring substituents. These compounds may be good fungicidal for the conservation of celluloid materials lying in the museum. Key words: Organoborates, Silver metaborate, Acyl chloride, spore, Antifungal. Introduction All organoborates are synthesized with the used of silver metaborate (AgBO2) and boric acid [1] with organic halides such as 3,5, dinitrobenzoyl chloride and p-nitrobenzoyl chloride. Since the silver atom in AgBO2 in photo activated state the reaction with organic halides having a polarized halogen atom will readily takes place with the formation of silver halides. Steric effect is an important factor in determining the course of reactions well as the stability of the product. It is observed that acyl chloride reacts more readily than sterically hindered pyrollium chloride [2]. The reaction of silver metaborate with organic halides may be ascribed to an ionic mechanism. Indeed acyllium [3] ion have been recognized as possible intermediate in acid catalyzed etherification in Friedel- Crafts reactions through the universality of such mechanism in doubt and the ion is assumed to resonate between the below structures: R – C O+ R – C+ O The presence of CO group polarizes the carbon halogen bond to great extent. -

The Effects of Borax on Milk Yield and Selected Metabolic Parameters in Austrian Simmental (Fleckvieh) Cows
Veterinarni Medicina, 60, 2015 (4): 175–180 Original Paper doi: 10.17221/8104-VETMED The effects of borax on milk yield and selected metabolic parameters in Austrian Simmental (Fleckvieh) cows M. Kabu, C. Uyarlar Faculty of Veterinary Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey ABSTRACT: This study was conducted to determine the effects of orally administered borax on milk yield and on several blood variables related to metabolism in early lactation in Austrian Simmental cows (Fleckvieh). Twenty primiparous cows were selected at parturition and then assigned to one of two groups, the control group or the borax group. The study lasted for four weeks. Borax was administered orally at 0.2 mg/kg/day (Boron group) to all treatment cows shortly after the noon milking, whereas cows in the control group were not treated. All cows consumed the same diet. All feeds in the diet were analysed for crude cellulose, protein, ether extract, ash, and dry matter according to the Weende Analysis Systems, in addition to ADF and NDF, according to Van Soest. Blood samples were collected from all cows via the vena jugularis on lactation Days 0, 7, 15, 21 and 28 and ana- lysed for the following: serum boron (B), non-esterified fatty acids (NEFA), beta-hydroxybutyric acid (BHBA), total cholesterol (TChol), high density lipids (HDL), total protein (TP), blood urea nitrogen (BUN), albumin (ALB), creatine (CRE), uric acid (UA), glucose (GLU), aspartate aminotransferase (AST), alanine aminotrans- ferase (ALT) and gamma-glutamyl transpeptidase (GGT) concentrations. Serum B concentration was higher in the borax group than in the control group at Weeks 1, 2, 3, and 4 of the experiment. -

Chemical Pretreatment for RO and NF
Chemical Pretreatment For RO and NF October 2013 Lenntech [email protected] Tel. +31-152-610-900 www.lenntech.com Fax. +31-152-616-289 There are a number of chemicals that can be introduced into the RO feed to enhance the operation of the RO system. Acids Caustic Dechlorination chemicals Antiscalants and Dispersants Acids: Acids, typically hydrochloric [HCl] or sulfuric [H2SO4], are injected into the RO feed to lower pH. Sulfuric acid is used more often than HCl acid. One reason for this is because sulfuric acid is relatively lower in operating cost than HCl acid. Another advantage to using sulfuric over HCl is the reduced fuming to the atmosphere, which means less corrosion to surrounding metal components. Sulfuric acid is sometimes preferred over HCl since there is a better membrane rejection of the sulfate ion than the chloride ion. Technical grade sulfuric acid, with no other additives, is suitable for use with a RO. Sulfuric acid is commercially available as a 20% and 93% solution. The 93% solution is also referred to as “66 0 Baume solution”. Caution is required in diluting 93% sulfuric acid, since the maximum heat of dilution of about 280 F occurs around 60%. It is critical that the concentrated acid is added slowly to the top of dilution water that is being agitated to minimize the buildup of heat and boiling of the makeup solution. Hydrochloric acid is preferred when calcium sulfate, barium sulfate, or strontium sulfate scaling is a concern. Sulfuric acid increases the sulfate ion level in the RO feed, which directly increases the potential for sulfate-based scaling. -

Available At
Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 VOL. IV.—1890. c THE JOURNAL OF LARYN GO LOGY AND RHINOLOGY; AN ANALYTICAL RECORD OF CURRENT LITERATURE RELATING TO THE THROAT AND NOSE. EDITED BY SIR MORELL MACKENZIE, M.D. Lond., AM) R. NORRIS WOLFENDEN, M.D. Cantab., WITH TUT'. CO-OI'EKAl IuN OF DR. JOHN N. MACKENZIE (BALTIMORE) DR. VALERIUS IDELSON (BERNE) DR. BRYSON DELAVAN (NEW YORK) DR. MICHAEL (HAMBURG) DR. W. PORTER (ST. LOUIS) PROF. RAMON DE LA SOTA Y LASTRA DR. JOAL (MONT DORE) (SEVILLE) PROF. MASSEI (NAPLES) DR. HOLGER MYGIND (COPENHAGEN) PROF. GUYE (AMSTERDAM) DR. SMYLY (DUBLIN) DR. CAPART (BRUSSELS) DR. DAVID COLLINGWOOD (SYDNEY; DR. CONSTANTIN KARWOWSKI (WARSAW) DR. G. W. MAJOR (MONTREAL) DR. BARCLAY J. BARON (BRISTOL) DR. MAXWELL ROSS (EDINBURGH) Dr. G. HUNTER MACKENZIE (Edinburgh). F. A. DAVIS, LONDON: PHILADELPHIA: 40, BERNERS STREET, \V. 1231, FILBERT STREET. Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 CONTENTS. -

Sodium Hydrogen Sulfate
SODIUM HYDROGEN SULFATE New specifications prepared at the 68th JECFA (2007), published in FAO JECFA Monographs 4 (2007). No ADI has been allocated to sodium hydrogen sulfate for use in production of acidified sodium chlorite. An ADI “not specified’ was established for sodium sulfate at the 57th JECFA (2001) and no ADI was allocated for sulfuric acid at the 20th JECFA (1976). SYNONYMS Sodium acid sulfate; nitre cake; sodium bisulfate; sulfuric acid, monosodium salt. DEFINITION Sodium chloride and sulfuric acid are combined at elevated temperatures to produce molten sodium hydrogen sulfate. The molten sodium hydrogen sulfate is sprayed and cooled to form a solid product with uniform particle size. C.A.S. number 7681-38-1 Chemical formula NaHSO4 Formula weight 120.06 Structural Formula O- OH Na + S O O Assay Not less than 85% DESCRIPTION White crystals or granules FUNCTIONAL USES For use in antimicrobial washing solutions CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Freely soluble in water Sodium (Vol. 4) Passes test Sulfate (Vol. 4) Passes test PURITY Loss on drying (Vol.4) Not more than 0.8% (105o, 3h, use 25 g of sample), Water-insoluble matter Not more than 0.05% (Use 50 g of sample and 300 ml hot water) (Vol. 4) Lead (Vol. 4) Not more than 2 mg/kg Determine using an AAS/ICP-AES technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on principles of methods described in Volume 4 (under “General Methods, Metallic Impurities”). Selenium (Vol. 4) Not more than 5 mg/kg Determine using an AAS/ICP-AES technique appropriate to the specified level. -

The Influence of the Presence of Borax and Nacl on Water
sensors Letter The Influence of the Presence of Borax and NaCl on Water Absorption Pattern during Sturgeon Caviar (Acipenser transmontanus) Storage Massimo Brambilla 1 , Marina Buccheri 2, Maurizio Grassi 2 , Annamaria Stellari 1, Mario Pazzaglia 3, Elio Romano 1 and Tiziana M. P. Cattaneo 2,* 1 Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria–CREA–Centro di Ricerca Ingegneria e Trasformazioni Agroalimentari, Via Milano, 43–24047 Treviglio (Bergamo), Italy; [email protected] (M.B.); [email protected] (A.S.); [email protected] (E.R.) 2 Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria–CREA–Centro di Ricerca Ingegneria e Trasformazioni Agroalimentari, Via G. Venezian, 26–20133 Milano, Italy; [email protected] (M.B.); [email protected] (M.G.) 3 Agroittica Lombarda S.p.A., Via Kennedy, 25012 Calvisano Brescia, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-02-239-557-212 Received: 10 November 2020; Accepted: 10 December 2020; Published: 15 December 2020 Abstract: Sturgeon caviar quality relies not only on the perfect dosage of the ingredients but also on the long sturgeon breeding cycle (about 12–15 years) and the exact timing of the egg extraction. For the improvement and the promotion of Italian caviar, the development of an analytical system dedicated to fish products, and caviar, in particular, is fundamental. The use of near-infrared spectrometry (NIRS) technology is auspicious. The aquaphotomics approach proved to be an adequate analytical tool to highlight, in real-time, the differences in caviar quality stored with, or without, borax as a preservative.