Richmond City Health District
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit: Stoichiometry Lesson: #10 Topics: Solvay Process
Unit: Stoichiometry Lesson: #10 Topics: Solvay Process The Solvay Process: A Case Study Many important industrial processes involve chemical reactions that are difficult to perform and still be economically feasible. In some cases, the overall process must follow a series of reaction steps. Sodium carbonate is a chemical that is needed in large amounts – it is used in the production of glass and soap. Historically, sodium carbonate was produced by burning plants or coal and mixing the ashes with water This method was both inefficient and very expensive because of the large amount of material that needed to be burned. The Solvay process was developed as a cost effective alternative. It involves a series of reaction steps and has a net reaction of: CaCO3 (s) + 2 NaCl (aq) Na2CO3 (aq) + CaCl2 (aq) A net reaction is determined by looking at the various reaction steps and eliminating any chemical that appears as both a reactant and a product in the various steps. These chemicals are known as intermediates. Step 1: Calcium carbonate (limestone) is decomposed by heat to form calcium oxide (lime) and carbon dioxide. CaCO3 (s) CaO (s) + CO2 (g) Step 2: Carbon dioxide reacts with aqueous ammonia and water to form aqueous ammonium hydrogen carbonate. CO2 (g) + NH3 (aq) + H2O (l) NH4HCO3 (aq) Step 3: The aqueous ammonium hydrogen carbonate reacts with sodium chloride to produce ammonium chloride and solid baking soda. NH4HCO3 (aq) + NaCl (aq) NH4Cl (aq) + NaHCO3 (s) Step 4: The baking soda is heated up and decomposed into sodium carbonate, water vapor and carbon dioxide. 2 NaHCO3 (s) Na2CO3 (s) + H2O (g) + CO2 (g) Step 5: The lime that was produced in the first step is mixed with water to produce slaked lime (calcium hydroxide). -

Living up to Life Special Stain Kit Modified Grocott's Methenamine
Living up to Life Special Stain Kit Modified Grocott’s Methenamine Silver Stain Catalog No: 38016SS12 Intended Use 16. Rinse slides briefly in deionized water. For In Vitro Diagnostic Use. For Laboratory Use. 17. Dehydrate slides in three changes of absolute alcohol. The reagents in this kit are intended for “In Vitro“ use only. The Modified Grocott’s Methenamine 18. Clear slides in two changes of xylene and mount in a xylene miscible medium. Silver Stain when used with appropriate histological protocols may be used for the demonstration of defined fungi and infectious agents such as Aspergillus sp., Pneumocystis Staining Protocol (Microwave) carinii and Cryptococcus neoformans in formalin fixed, paraffin embedded tissue sections. Exercise caution when using the microwave oven to heat any solution or reagent. The microwave must be properly ventilated to prevent the accumulation of fumes in the laboratory. Probable Mode of Action Microwave transparent Coplin jars and caps should be used during the staining process. The The mechanism of action of Modified Grocott’s Methenamine Silver Stain is based upon the caps should be loosely attached to prevent spills. Caps with ventilation holes also may be used. capacity of aldehyde groups to reduce cationic silver (Ag+) to metallic silver. Chromic acid is All microwave ovens should be used in accordance with the manufacturer’s instructions. The used to generate aldehyde groups by the oxidation of 1-2 glycol groups within polysaccharide procedures described here were performed using an Energy Beam Sciences H2250 laboratory rich tissue components, e.g. glycogen, mucin, reticulin and fungal cell walls. When cationic microwave. -

The Reaction of Calcium Chloride with Carbonate Salts
The Reaction of Calcium Chloride with Carbonate Salts PRE-LAB ASSIGNMENT: Reading: Chapter 3 & Chapter 4, sections 1-3 in Brown, LeMay, Bursten, & Murphy. 1. What product(s) might be expected to form when solid lithium carbonate is added to an aqueous solution of calcium chloride? Write a balanced chemical equation for this process. 2. How many grams of lithium carbonate would you need to fully react with one mole of calcium chloride? Please show your work. INTRODUCTION: The purpose of this lab is to help you discover the relationships between the reactants and products in a precipitation reaction. In this lab you will react a calcium chloride solution with lithium carbonate, sodium carbonate, or potassium carbonate. The precipitate that results will be filtered and weighed. In each determination you will use the same amount of calcium chloride and different amounts of your carbonate salt. This experiment is a "discovery"- type experiment. The procedure will be carefully described, but the analysis of the data is left purposely vague. You will work in small groups to decide how best to work up the data. In the process you will have the chance to discover some principles, to use what you have learned in lecture, and to practice thinking about manipulative details and theory at the same time. Plotting your data in an appropriate manner should verify the identity of the precipitate and clarify the relationship between the amount of carbonate salt and the yield of precipitate. Predicting the formulas of ionic compounds. Compounds like calcium chloride (CaCl2) and sodium carbonate (Na2CO3) are ionic substances. -

Scientific Committee on Consumer Safety SCCS
SCCS/1249/09 Revision of 28 September 2010 Scientific Committee on Consumer Safety SCCS OPINION ON Boron compounds The SCCS adopted this opinion at its 7th plenary meeting of 22 June 2010 SCCS/1249/09 Opinion on boron compounds ___________________________________________________________________________________________ About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Jürgen Angerer, Ulrike Bernauer, Claire Chambers, Qasim Chaudhry, Gisela Degen, Gerhard Eisenbrand, Thomas Platzek, Suresh Chandra Rastogi, Vera Rogiers, Christophe Rousselle, Tore Sanner, Kai Savolainen, Jacqueline Van Engelen, Maria Pilar Vinardell, Rosemary Waring, Ian R. -

Sodium Laurilsulfate Used As an Excipient
9 October 2017 EMA/CHMP/351898/2014 corr. 1* Committee for Human Medicinal Products (CHMP) Sodium laurilsulfate used as an excipient Report published in support of the ‘Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use’ (EMA/CHMP/606830/2017) * Deletion of the E number. Please see the corrected Annex for further details. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Sodium laurilsulfate used as an excipient Table of contents Executive summary ..................................................................................... 3 Introduction ................................................................................................ 4 Scientific discussion .................................................................................... 4 1. Characteristics ....................................................................................... 4 1.1 Category (function) ............................................................................................. 4 1.2 Properties........................................................................................................... 4 1.3 Use in medicinal products ..................................................................................... 5 1.4 Regulatory -
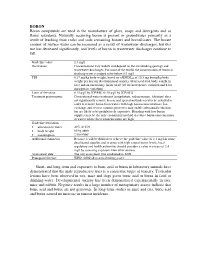
BORON Boron Compounds Are Used in the Manufacture of Glass, Soaps and Detergents and As Flame Retardants
BORON Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall. Guideline value 2.4 mg/l Occurrence Concentrations vary widely and depend on the surrounding geology and wastewater discharges. For most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l. TDI 0.17 mg/kg body weight, based on a BMDL 05 of 10.3 mg boron/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) Limit of detection 0.15 µg/l by ICP/MS; 6–10 µg/l by ICP/AES Treatment performance Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be installed in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. Guideline derivation • allocation to water 40% of TDI • body weight 60 kg adult • consumption 2 litres/day Additional comments Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. -

STUDENTS 2 SCIENCE Virtual Lab Experiment Precipitates
STUDENTS 2 SCIENCE Virtual Lab Experiment Precipitates An investigation of solubility and precipitation. A classroom Experiment in Kit Form for Grades 9-12 Brief Background: Students will learn the difference between reaction precipitates like the precipitation of Basic copper carBonate from the reaction of copper chloride and sodium carBonate and those that result solely from a change in solution soluBility (water vs. isopropanol). They will learn aBout precipitation in water treatment and purification and aBout the scientists who work to provide or maintain clean water for us. Additionally, students will learn that precipitates can sometimes Be redissolved By adding another chemical compound and thus carrying out an additional chemical reaction. Safety Students and teachers must wear properly fitting goggles as they prepare for, conduct, and clean up from the activities in the kit. Read and follow all safety warnings. Also review the Materials Safety Data Sheets. Students must wash their hands with soap and water after the activities. The activities described in this kit are intended for students under the direct supervision of teachers. Student kit packaged as 13 individual units for a class size of 26. Each student package is shared by 2 students. Items in each 2-student Ziploc bag: • (1) vial labeled “CuSO4” approximately 1/4 filled with cupric sulfate • (1) empty vial laBeled “CuSO4 Solution” • (1) 4-mL vial laBeled “CaCl2” containing small amount calcium chloride • (1) vial labeled “NaHCO3” (in red lettering) approximately 1/3 -

Synthesis, Characterization and Fungicidal Effects of Some
Journal of Manmohan Memorial Institute of Health Sciences (JMMIHS) Synthesis, Characterization and Fungicidal Effects of some Organoborates Shaheen Akther1 Khanal DP2 Ullah SS3 Abstract Synthesis of benzoyl borate, phthasloyl borate and oxyl borate was done and characterized by infra red, ultraviolet and mass spectra. All newly synthesized and characterized organoborates were tested for their antifungal activity against pencillium that spoilt mostly celluloid materials stored in museum. All synthesized organoborates are unstable in room temperature and quickly degraded on exposure to air. Benzoyl borate found to be most active antifungal compound of the series against penicillium. The fungicidal activity increases in hydrophobicity and electron withdrawing nature of the benzene ring substituents. These compounds may be good fungicidal for the conservation of celluloid materials lying in the museum. Key words: Organoborates, Silver metaborate, Acyl chloride, spore, Antifungal. Introduction All organoborates are synthesized with the used of silver metaborate (AgBO2) and boric acid [1] with organic halides such as 3,5, dinitrobenzoyl chloride and p-nitrobenzoyl chloride. Since the silver atom in AgBO2 in photo activated state the reaction with organic halides having a polarized halogen atom will readily takes place with the formation of silver halides. Steric effect is an important factor in determining the course of reactions well as the stability of the product. It is observed that acyl chloride reacts more readily than sterically hindered pyrollium chloride [2]. The reaction of silver metaborate with organic halides may be ascribed to an ionic mechanism. Indeed acyllium [3] ion have been recognized as possible intermediate in acid catalyzed etherification in Friedel- Crafts reactions through the universality of such mechanism in doubt and the ion is assumed to resonate between the below structures: R – C O+ R – C+ O The presence of CO group polarizes the carbon halogen bond to great extent. -

CASE STUDY Methane Recovery at Non-Coal Mines
CASE STUDY Methane Recovery at Non-coal Mines Background Existing methane recovery projects at non-coal mines reduce greenhouse gas (GHG) emissions by up to 3 million tons of carbon dioxide equivalent (CO₂e). Although methane emissions are generally associated with coal mines, methane is also found in salt, potash, trona, diamond, gold, base metals, and lead mines throughout the world. Many of these deposits are located adjacent to hydrocarbon (e.g., coal and oil) deposits where methane originates. Consequently, during the process of mining non-coal deposits, methane may be released. Overview of Methane Recovery Projects Beatrix Gold Mine, South Africa Promethium Carbon, a carbon and climate change advisory firm, has developed a project to capture and use methane from the Beatrix Gold Mine in Free State, South Africa (see box). Project Specifications: Beatrix Gold Mine Name Capture and Utilization of Methane at the Gold Fields–owned Beatrix Mine in South Africa Site Free State Province, South Africa Owner Gold Fields Ltd., Exxaro On-site Pty Ltd. Dates of Operation May 2011–present Equipment Phase 1 • GE Roots Blowers • Trolex gas monitoring systems • Flame arrestor • 23 burner flare Equipment Phase 2 Addition of internal combustion engines End-use Phase 1 Destruction through flaring End-use Phase 2 4 MW power generation Emission Reductions 1.7 million tons CO²e expected over CDM project lifetime (through 2016) Commissioning of Flare at Beatrix Gold Mine (Photo courtesy of Gold Fields Ltd.) CASE STUDY Methane Recovery at Non-coal Mines ABUTEC Mine Gas Incinerator at Green River Trona Mine (Photo courtesy of Solvay Chemicals, Inc.) Green River Trona Mine, United States Project Specifications: Green River Trona Mine Trona is an evaporite mineral mined in the United States Name Green River Trona Mine Methane as the primary source of sodium carbonate, or soda ash. -

The Effects of Borax on Milk Yield and Selected Metabolic Parameters in Austrian Simmental (Fleckvieh) Cows
Veterinarni Medicina, 60, 2015 (4): 175–180 Original Paper doi: 10.17221/8104-VETMED The effects of borax on milk yield and selected metabolic parameters in Austrian Simmental (Fleckvieh) cows M. Kabu, C. Uyarlar Faculty of Veterinary Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey ABSTRACT: This study was conducted to determine the effects of orally administered borax on milk yield and on several blood variables related to metabolism in early lactation in Austrian Simmental cows (Fleckvieh). Twenty primiparous cows were selected at parturition and then assigned to one of two groups, the control group or the borax group. The study lasted for four weeks. Borax was administered orally at 0.2 mg/kg/day (Boron group) to all treatment cows shortly after the noon milking, whereas cows in the control group were not treated. All cows consumed the same diet. All feeds in the diet were analysed for crude cellulose, protein, ether extract, ash, and dry matter according to the Weende Analysis Systems, in addition to ADF and NDF, according to Van Soest. Blood samples were collected from all cows via the vena jugularis on lactation Days 0, 7, 15, 21 and 28 and ana- lysed for the following: serum boron (B), non-esterified fatty acids (NEFA), beta-hydroxybutyric acid (BHBA), total cholesterol (TChol), high density lipids (HDL), total protein (TP), blood urea nitrogen (BUN), albumin (ALB), creatine (CRE), uric acid (UA), glucose (GLU), aspartate aminotransferase (AST), alanine aminotrans- ferase (ALT) and gamma-glutamyl transpeptidase (GGT) concentrations. Serum B concentration was higher in the borax group than in the control group at Weeks 1, 2, 3, and 4 of the experiment. -

Crystals of an Insoluble Carbonate of Copper Grown Under a Soda Solution L
Journal of the Arkansas Academy of Science Volume 1 Article 22 1941 Crystals of an Insoluble Carbonate of Copper Grown under a Soda Solution L. B. Roberts University of Arkansas at Monticello Edwin Roberts University of Arkansas at Monticello Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Organic Chemistry Commons Recommended Citation Roberts, L. B. and Roberts, Edwin (1941) "Crystals of an Insoluble Carbonate of Copper Grown under a Soda Solution," Journal of the Arkansas Academy of Science: Vol. 1 , Article 22. Available at: http://scholarworks.uark.edu/jaas/vol1/iss1/22 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 1 [1941], Art. 22 CRYSTALS OF AN INSOLUBLE CARBONATE OF COPPER GROWN UNDER A SODA SOLUTION1 L. B. Roberts and Edwin Roberts, Arkansas Agricultural and Mechanical College, Monticello When an old fire extinguisher of the soda-acid type was opened and emptied preparatory to recharging, several grams of blue crystals were found in the bottom of the container. -

Safety Data Sheet According to 29CFR1910/1200 and GHS Rev
Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 12.12.2014 Page 1 of 7 Sodium Carbonate,Anhydrous SECTION 1 : Identification of the substance/mixture and of the supplier Product name : Sodium Carbonate,Anhydrous Manufacturer/Supplier Trade name: Manufacturer/Supplier Article number: S25539D Recommended uses of the product and uses restrictions on use: Manufacturer Details: AquaPhoenix Scientific 9 Barnhart Drive, Hanover, PA 17331 Supplier Details: Fisher Science Education 15 Jet View Drive, Rochester, NY 14624 Emergency telephone number: Fisher Science Education Emergency Telephone No.: 800-535-5053 SECTION 2 : Hazards identification Classification of the substance or mixture: Irritant Eye irritation, category 2A Eye Irritation 2 Signal word :Warning Hazard statements: Causes serious eye irritation Precautionary statements: If medical advice is needed, have product container or label at hand Keep out of reach of children Read label before use Do not eat, drink or smoke when using this product Wear protective gloves/protective clothing/eye protection/face protection Wash skin thoroughly after handling IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing If eye irritation persists get medical advice/attention Other Non-GHS Classification: WHMIS D2B E Created by Global Safety Management, Inc. -Tel: 1-813-435-5161 - www.gsmsds.com Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 12.12.2014 Page 2 of 7 Sodium Carbonate,Anhydrous NFPA/HMIS NFPA SCALE (0-4) HMIS RATINGS (0-4) SECTION 3 : Composition/information on ingredients Ingredients: CAS 497-19-8 Sodium Carbonate, Anhydrous 100 % Percentages are by weight SECTION 4 : First aid measures Description of first aid measures After inhalation: Move exposed individual to fresh air.