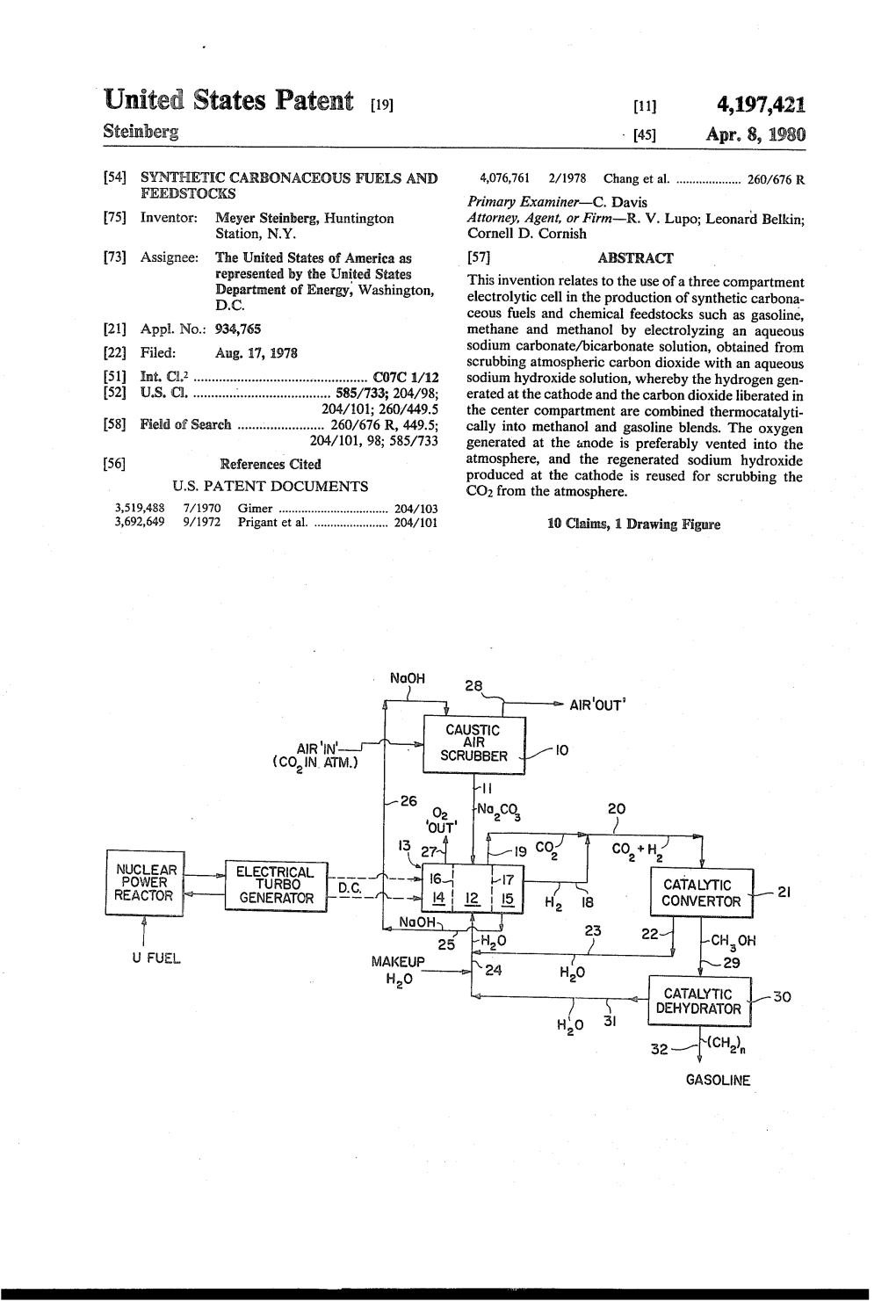
Ca-Sic O (Coin Atm.) Scrubber
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit: Stoichiometry Lesson: #10 Topics: Solvay Process
Unit: Stoichiometry Lesson: #10 Topics: Solvay Process The Solvay Process: A Case Study Many important industrial processes involve chemical reactions that are difficult to perform and still be economically feasible. In some cases, the overall process must follow a series of reaction steps. Sodium carbonate is a chemical that is needed in large amounts – it is used in the production of glass and soap. Historically, sodium carbonate was produced by burning plants or coal and mixing the ashes with water This method was both inefficient and very expensive because of the large amount of material that needed to be burned. The Solvay process was developed as a cost effective alternative. It involves a series of reaction steps and has a net reaction of: CaCO3 (s) + 2 NaCl (aq) Na2CO3 (aq) + CaCl2 (aq) A net reaction is determined by looking at the various reaction steps and eliminating any chemical that appears as both a reactant and a product in the various steps. These chemicals are known as intermediates. Step 1: Calcium carbonate (limestone) is decomposed by heat to form calcium oxide (lime) and carbon dioxide. CaCO3 (s) CaO (s) + CO2 (g) Step 2: Carbon dioxide reacts with aqueous ammonia and water to form aqueous ammonium hydrogen carbonate. CO2 (g) + NH3 (aq) + H2O (l) NH4HCO3 (aq) Step 3: The aqueous ammonium hydrogen carbonate reacts with sodium chloride to produce ammonium chloride and solid baking soda. NH4HCO3 (aq) + NaCl (aq) NH4Cl (aq) + NaHCO3 (s) Step 4: The baking soda is heated up and decomposed into sodium carbonate, water vapor and carbon dioxide. 2 NaHCO3 (s) Na2CO3 (s) + H2O (g) + CO2 (g) Step 5: The lime that was produced in the first step is mixed with water to produce slaked lime (calcium hydroxide). -

The Reaction of Calcium Chloride with Carbonate Salts
The Reaction of Calcium Chloride with Carbonate Salts PRE-LAB ASSIGNMENT: Reading: Chapter 3 & Chapter 4, sections 1-3 in Brown, LeMay, Bursten, & Murphy. 1. What product(s) might be expected to form when solid lithium carbonate is added to an aqueous solution of calcium chloride? Write a balanced chemical equation for this process. 2. How many grams of lithium carbonate would you need to fully react with one mole of calcium chloride? Please show your work. INTRODUCTION: The purpose of this lab is to help you discover the relationships between the reactants and products in a precipitation reaction. In this lab you will react a calcium chloride solution with lithium carbonate, sodium carbonate, or potassium carbonate. The precipitate that results will be filtered and weighed. In each determination you will use the same amount of calcium chloride and different amounts of your carbonate salt. This experiment is a "discovery"- type experiment. The procedure will be carefully described, but the analysis of the data is left purposely vague. You will work in small groups to decide how best to work up the data. In the process you will have the chance to discover some principles, to use what you have learned in lecture, and to practice thinking about manipulative details and theory at the same time. Plotting your data in an appropriate manner should verify the identity of the precipitate and clarify the relationship between the amount of carbonate salt and the yield of precipitate. Predicting the formulas of ionic compounds. Compounds like calcium chloride (CaCl2) and sodium carbonate (Na2CO3) are ionic substances. -

Sodium Laurilsulfate Used As an Excipient
9 October 2017 EMA/CHMP/351898/2014 corr. 1* Committee for Human Medicinal Products (CHMP) Sodium laurilsulfate used as an excipient Report published in support of the ‘Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use’ (EMA/CHMP/606830/2017) * Deletion of the E number. Please see the corrected Annex for further details. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Sodium laurilsulfate used as an excipient Table of contents Executive summary ..................................................................................... 3 Introduction ................................................................................................ 4 Scientific discussion .................................................................................... 4 1. Characteristics ....................................................................................... 4 1.1 Category (function) ............................................................................................. 4 1.2 Properties........................................................................................................... 4 1.3 Use in medicinal products ..................................................................................... 5 1.4 Regulatory -

STUDENTS 2 SCIENCE Virtual Lab Experiment Precipitates
STUDENTS 2 SCIENCE Virtual Lab Experiment Precipitates An investigation of solubility and precipitation. A classroom Experiment in Kit Form for Grades 9-12 Brief Background: Students will learn the difference between reaction precipitates like the precipitation of Basic copper carBonate from the reaction of copper chloride and sodium carBonate and those that result solely from a change in solution soluBility (water vs. isopropanol). They will learn aBout precipitation in water treatment and purification and aBout the scientists who work to provide or maintain clean water for us. Additionally, students will learn that precipitates can sometimes Be redissolved By adding another chemical compound and thus carrying out an additional chemical reaction. Safety Students and teachers must wear properly fitting goggles as they prepare for, conduct, and clean up from the activities in the kit. Read and follow all safety warnings. Also review the Materials Safety Data Sheets. Students must wash their hands with soap and water after the activities. The activities described in this kit are intended for students under the direct supervision of teachers. Student kit packaged as 13 individual units for a class size of 26. Each student package is shared by 2 students. Items in each 2-student Ziploc bag: • (1) vial labeled “CuSO4” approximately 1/4 filled with cupric sulfate • (1) empty vial laBeled “CuSO4 Solution” • (1) 4-mL vial laBeled “CaCl2” containing small amount calcium chloride • (1) vial labeled “NaHCO3” (in red lettering) approximately 1/3 -

CASE STUDY Methane Recovery at Non-Coal Mines
CASE STUDY Methane Recovery at Non-coal Mines Background Existing methane recovery projects at non-coal mines reduce greenhouse gas (GHG) emissions by up to 3 million tons of carbon dioxide equivalent (CO₂e). Although methane emissions are generally associated with coal mines, methane is also found in salt, potash, trona, diamond, gold, base metals, and lead mines throughout the world. Many of these deposits are located adjacent to hydrocarbon (e.g., coal and oil) deposits where methane originates. Consequently, during the process of mining non-coal deposits, methane may be released. Overview of Methane Recovery Projects Beatrix Gold Mine, South Africa Promethium Carbon, a carbon and climate change advisory firm, has developed a project to capture and use methane from the Beatrix Gold Mine in Free State, South Africa (see box). Project Specifications: Beatrix Gold Mine Name Capture and Utilization of Methane at the Gold Fields–owned Beatrix Mine in South Africa Site Free State Province, South Africa Owner Gold Fields Ltd., Exxaro On-site Pty Ltd. Dates of Operation May 2011–present Equipment Phase 1 • GE Roots Blowers • Trolex gas monitoring systems • Flame arrestor • 23 burner flare Equipment Phase 2 Addition of internal combustion engines End-use Phase 1 Destruction through flaring End-use Phase 2 4 MW power generation Emission Reductions 1.7 million tons CO²e expected over CDM project lifetime (through 2016) Commissioning of Flare at Beatrix Gold Mine (Photo courtesy of Gold Fields Ltd.) CASE STUDY Methane Recovery at Non-coal Mines ABUTEC Mine Gas Incinerator at Green River Trona Mine (Photo courtesy of Solvay Chemicals, Inc.) Green River Trona Mine, United States Project Specifications: Green River Trona Mine Trona is an evaporite mineral mined in the United States Name Green River Trona Mine Methane as the primary source of sodium carbonate, or soda ash. -

Crystals of an Insoluble Carbonate of Copper Grown Under a Soda Solution L
Journal of the Arkansas Academy of Science Volume 1 Article 22 1941 Crystals of an Insoluble Carbonate of Copper Grown under a Soda Solution L. B. Roberts University of Arkansas at Monticello Edwin Roberts University of Arkansas at Monticello Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Organic Chemistry Commons Recommended Citation Roberts, L. B. and Roberts, Edwin (1941) "Crystals of an Insoluble Carbonate of Copper Grown under a Soda Solution," Journal of the Arkansas Academy of Science: Vol. 1 , Article 22. Available at: http://scholarworks.uark.edu/jaas/vol1/iss1/22 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 1 [1941], Art. 22 CRYSTALS OF AN INSOLUBLE CARBONATE OF COPPER GROWN UNDER A SODA SOLUTION1 L. B. Roberts and Edwin Roberts, Arkansas Agricultural and Mechanical College, Monticello When an old fire extinguisher of the soda-acid type was opened and emptied preparatory to recharging, several grams of blue crystals were found in the bottom of the container. -

Safety Data Sheet According to 29CFR1910/1200 and GHS Rev
Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 12.12.2014 Page 1 of 7 Sodium Carbonate,Anhydrous SECTION 1 : Identification of the substance/mixture and of the supplier Product name : Sodium Carbonate,Anhydrous Manufacturer/Supplier Trade name: Manufacturer/Supplier Article number: S25539D Recommended uses of the product and uses restrictions on use: Manufacturer Details: AquaPhoenix Scientific 9 Barnhart Drive, Hanover, PA 17331 Supplier Details: Fisher Science Education 15 Jet View Drive, Rochester, NY 14624 Emergency telephone number: Fisher Science Education Emergency Telephone No.: 800-535-5053 SECTION 2 : Hazards identification Classification of the substance or mixture: Irritant Eye irritation, category 2A Eye Irritation 2 Signal word :Warning Hazard statements: Causes serious eye irritation Precautionary statements: If medical advice is needed, have product container or label at hand Keep out of reach of children Read label before use Do not eat, drink or smoke when using this product Wear protective gloves/protective clothing/eye protection/face protection Wash skin thoroughly after handling IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing If eye irritation persists get medical advice/attention Other Non-GHS Classification: WHMIS D2B E Created by Global Safety Management, Inc. -Tel: 1-813-435-5161 - www.gsmsds.com Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 12.12.2014 Page 2 of 7 Sodium Carbonate,Anhydrous NFPA/HMIS NFPA SCALE (0-4) HMIS RATINGS (0-4) SECTION 3 : Composition/information on ingredients Ingredients: CAS 497-19-8 Sodium Carbonate, Anhydrous 100 % Percentages are by weight SECTION 4 : First aid measures Description of first aid measures After inhalation: Move exposed individual to fresh air. -

Disposal of Solid Chemicals in the Normal Trash
Disposal of Solid Chemicals in the Normal Trash Many solid chemicals can be safety discarded into the normal trash, provided they are in containers that are not broken or cracked and have tightly fitting caps. These chemicals are considered acceptable for ordinary disposal because they display none of the properties of hazardous waste, are of low acute toxicity, and have not been identified as having any chronic toxic effects as summarized in the National Institute of Occupational Safety and Health (NIOSH) “Registry of Toxic Effects of Chemical Substances”. Examples of chemicals acceptable for disposal as regular trash are listed below. To dispose of these chemicals, place the containers in a box lined with a plastic bag, tape the top of the box shut, write “Normal Trash” on the box and then place the box next to the lab trash container. Only solid forms of these chemicals can be disposed in this manner. Any questions about these chemicals or other chemicals that may be disposed of in the normal trash should be directed to the Hazardous Materials Technician (610) 330-5225. Chemicals Generally Acceptable for Disposal as Regular Trash Acacia powder, gum Detergent (most) Methyl salicylate Sodium carbonate arabic Cation exchange resins Methylene blue Sodium chloride Acid, Ascorbic Chromatographic Methyl stearate Sodium citrate Acid, Benzoic absorbents Nutrient agar Sodium dodecyl sulfate Acid, Boric Crystal violet Octacosane (SDS) Acid, Casamind Dextrin Parafin Sodium formate Acid, Citric Dextrose Pepsin Sodium iodide Acid, Lactic Diatomaceous -

SERELISA Paratb Ab Mono Indirect MSDS.Pdf
Issue date: 28-December-2016 Version number: 01 SAFETY DATA SHEET 1. Identification Product identifier SERELISA® ParaTB Ab Mono Indirect Other means of identification Synonyms Mycobacterium paratuberculosis Antibody Test Kit * SERELISA ParaTB * USDA #5064.01 Recommended use of the chemical and restrictions on use Recommended use Veterinary product used as diagnostic aid Restrictions on use Not for human use Details of manufacturer or importer Company Name (AU) Zoetis Australia Pty Ltd ABN 94 156 476 425 Level 6, 5 Rider Boulevard Rhodes NSW 2138 AUSTRALIA Tel 1800 814 883 Fax (02) 8876 0444 Email [email protected] Emergency Phone 1800 814 883 (all hours) Police and Fire Brigade Dial 000 If ineffective Dial Poisons Information Centre (13 1126 from anywhere in Australia) 2. Hazard(s) identification Classification of the hazardous chemical Physical hazards Not classified. Health hazards Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Sensitization, skin Category 1 Reproductive toxicity Category 1A Environmental hazards Hazardous to the aquatic environment, acute Category 3 hazard Hazardous to the aquatic environment, Category 3 long-term hazard Label elements, including precautionary statements Hazard symbol(s) Health Exclamation hazard mark Signal word Danger Hazard Statement(s) Causes skin irritation. May cause an allergic skin reaction. Causes serious eye irritation. May damage fertility or the unborn child. Harmful to aquatic life with long lasting effects. Precautionary Statement(s) Prevention Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Avoid breathing mist or vapour. Wash thoroughly after handling. Contaminated work clothing should not be allowed out of the workplace. -

Lab 7: What Is That White Powder? Goals: the Goal of This Lab Is to Identify All Five of the Unknown White Powders You Are Given
Chemistry 108, Spring 2017 Lab 7: What is That White Powder? Goals: The goal of this lab is to identify all five of the unknown white powders you are given. Each of these powders can be superficially described as a “dry white powder”, but they have different chemical and physical properties. You will need to develop your own procedure for this lab by reading the material given in this hand out. You will show your procedure to an instructor and it must be accepted before you can begin the lab. The possibilities of the unknown solids are given in the table below. FORMULA NAME USE NaCl sodium chloride Table salt NaHCO3 sodium bicarbonate Baking soda C12H22O11 sucrose Table sugar C6H12O6 glucose/dextrose Sweetener CaSO4 calcium sulfate Plaster of Paris (C6H12O6)n cornstarch Thickener MgSO4·7H2O magnesium sulfate (hydrated) Epsom Salts Na2B4O7 sodium tetraborate Borax KHC4H4O6 potassium bitartrate Cream of Tartar CaCO3 calcium carbonate Chalks, antacids NaOH sodium hydroxide Lye, drain cleaner Na2CO3 sodium carbonate Washing soda Procedure: You will need to develop your own procedure for this lab by reading the material given in this hand out. You will show your procedure to an instructor and it must be accepted before you can begin the lab. NOTE: You do not have to carry out each test on each powder. Tests You Can Perform: I. Solubility A. Water Solubility Eight of the twelve possible powders are soluble in water (dissolve in water). The four that are insoluble are calcium sulfate, calcium carbonate, cornstarch, and potassium bitartrate. To test water solubility, add a pea-sized sample to a test tube, and about 5 mL of water, stopper and shake the test tube. -

Richmond City Health District
In the Bathroom In the Laundry Mold and mildew cleaners Richmond Make a concentrated solution of borax and water Fabric softener City Health and clean affected areas. Use 1/4 to 1/2 cup baking soda in rinse water. Healthy Homes Scrub mildew spots with baking soda or borax. Add 1 cup white vinegar or 1/4 cup baking soda District Sponge with white vinegar. during final rinse. Initiative Scrub with white vinegar and salt. Use 1/2 cup borax and half the amount of detergent per wash load Mix 2 parts baking soda to 1 part borax. Sprinkle Laundry bleach on surfaces, dampen and allow to sit for a time to Use 1/4 cup white vinegar in rinse water to brighten absorb odors. Scrub and dark clothing. Keep It Clean —Safe r rinse. Substitute 1/2 cup borax per wash load to whiten Dissolve 1/2 cup vinegar and whites and brighten colors and to remove spots. Household Cleaners 1/2 cup borax in warm wa- Laundry presoak ter. Soak heavily soiled items in warm water with 1/2 cup washing soda for 30 minutes. To clean mildew from a Rub soiled areas with mild liquid soap. shower curtain, machine wash with 1/4 cup soap Stain Pretreatment and 1/2 cup baking soda; add 1 cup of white vine- Make a paste of baking soda sand water and spread gar to the rinse cycle. on stain. Wet with white vinegar. Soak in soapy water Scouring powder (Kitchen use also) Spray starch Pour 1/2 cup of baking soda into a bowl, add For regular fabrics, dissolve 2 tablespoons cornstarch enough liquid detergent to make a texture-like in a pint of cold water; store in a spray bottle. -

Safety Data Sheet
G-Biosciences, St Louis, MO, USA | 1-800-628-7730 | 1-314-991-6034 | [email protected] A Geno Technology, Inc. (USA) brand name Safety Data Sheet Cat. # 786-32ZN Reversible Zinc Stain™ Size: 25 Mini gels think proteins! think G-Biosciences! www.GBiosciences.com Zinc Reagent I Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 07/14/2016 Revision date: 05/11/2017 Version: 7.1 SECTION 1: Identification 1.1. Identification Product form : Mixture Product name : Zinc Reagent I Product code : 110Z 1.2. Recommended use and restrictions on use No additional information available 1.3. Supplier Geno Technology, Inc./ G-Biosciences 9800 Page Avenue Saint Louis, 63132-1429 - United States T 800-628-7730 - F 314-991-1504 [email protected] - www.GBiosciences.com 1.4. Emergency telephone number Emergency number : Chemtrec 1-800-424-9300 (USA/Canada), +1-703-527-3887 (Intl) SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS US classification Serious eye damage/eye irritation Category 2 H319 Causes serious eye irritation Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : Signal word (GHS US) : Warning Hazard statements (GHS US) : H319 - Causes serious eye irritation Precautionary statements (GHS US) : P264 - Wash hands, forearms and face thoroughly after handling. P280 - Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338 - If in eyes: Rinse cautiously with water for several minutes.