Boron and Drinking Water Summary Boron Is a Naturally Occurring Element and Is Used in Many Consumer Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Living up to Life Special Stain Kit Modified Grocott's Methenamine
Living up to Life Special Stain Kit Modified Grocott’s Methenamine Silver Stain Catalog No: 38016SS12 Intended Use 16. Rinse slides briefly in deionized water. For In Vitro Diagnostic Use. For Laboratory Use. 17. Dehydrate slides in three changes of absolute alcohol. The reagents in this kit are intended for “In Vitro“ use only. The Modified Grocott’s Methenamine 18. Clear slides in two changes of xylene and mount in a xylene miscible medium. Silver Stain when used with appropriate histological protocols may be used for the demonstration of defined fungi and infectious agents such as Aspergillus sp., Pneumocystis Staining Protocol (Microwave) carinii and Cryptococcus neoformans in formalin fixed, paraffin embedded tissue sections. Exercise caution when using the microwave oven to heat any solution or reagent. The microwave must be properly ventilated to prevent the accumulation of fumes in the laboratory. Probable Mode of Action Microwave transparent Coplin jars and caps should be used during the staining process. The The mechanism of action of Modified Grocott’s Methenamine Silver Stain is based upon the caps should be loosely attached to prevent spills. Caps with ventilation holes also may be used. capacity of aldehyde groups to reduce cationic silver (Ag+) to metallic silver. Chromic acid is All microwave ovens should be used in accordance with the manufacturer’s instructions. The used to generate aldehyde groups by the oxidation of 1-2 glycol groups within polysaccharide procedures described here were performed using an Energy Beam Sciences H2250 laboratory rich tissue components, e.g. glycogen, mucin, reticulin and fungal cell walls. When cationic microwave. -

The Lithium, Boron and Beryllium Content Of
Published in Geochimica et Cosmochimica Acta 72, issue 22, 5475-5504, 2008 1 which should be used for any reference to this work The Lithium, Boron and Beryllium content of serpentinized peridotites from ODP Leg 209 (Sites 1272A and 1274A): Implications for lithium and boron budgets of oceanic lithosphere Flurin Vils a,*, Laure Pelletier a, Angelika Kalt a, Othmar Mu¨ntener b, Thomas Ludwig c a Institut de Ge´ologie et d’Hydroge´ologie, Universite´ de Neuchaˆtel, Rue Emile-Argand 11, CP 158, CH-2009 Neuchaˆtel, Switzerland b Institut de Mine´ralogie et Ge´ochimie, Universite´ de Lausanne, Anthropole, CH-1015 Lausanne, Switzerland c Mineralogisches Institut, Ruprecht-Karls-Universita¨t Heidelberg, Im Neuenheimer Feld 236, D-69120 Heidelberg, Germany Abstract Despite the key importance of altered oceanic mantle as a repository and carrier of light elements (B, Li, and Be) to depth, its inventory of these elements has hardly been explored and quantified. In order to constrain the systematics and budget of these elements we have studied samples of highly serpentinized (>50%) spinel harzburgite drilled at the Mid-Atlantic Ridge (Fifteen– Twenty Fracture zone, ODP Leg 209, Sites 1272A and 1274A). In-situ analysis by secondary ion mass spectrometry reveals that the B, Li and Be contents of mantle minerals (olivine, orthopyroxene, and clinopyroxene) remain unchanged during serpent- inization. B and Li abundances largely correspond to those of unaltered mantle minerals whereas Be is close to the detection limit. The Li contents of clinopyroxene are slightly higher (0.44–2.8 lggÀ1) compared to unaltered mantle clinopyroxene, and olivine and clinopyroxene show an inverse Li partitioning compared to literature data. -

National Primary Drinking Water Regulations
National Primary Drinking Water Regulations Potential health effects MCL or TT1 Common sources of contaminant in Public Health Contaminant from long-term3 exposure (mg/L)2 drinking water Goal (mg/L)2 above the MCL Nervous system or blood Added to water during sewage/ Acrylamide TT4 problems; increased risk of cancer wastewater treatment zero Eye, liver, kidney, or spleen Runoff from herbicide used on row Alachlor 0.002 problems; anemia; increased risk crops zero of cancer Erosion of natural deposits of certain 15 picocuries Alpha/photon minerals that are radioactive and per Liter Increased risk of cancer emitters may emit a form of radiation known zero (pCi/L) as alpha radiation Discharge from petroleum refineries; Increase in blood cholesterol; Antimony 0.006 fire retardants; ceramics; electronics; decrease in blood sugar 0.006 solder Skin damage or problems with Erosion of natural deposits; runoff Arsenic 0.010 circulatory systems, and may have from orchards; runoff from glass & 0 increased risk of getting cancer electronics production wastes Asbestos 7 million Increased risk of developing Decay of asbestos cement in water (fibers >10 fibers per Liter benign intestinal polyps mains; erosion of natural deposits 7 MFL micrometers) (MFL) Cardiovascular system or Runoff from herbicide used on row Atrazine 0.003 reproductive problems crops 0.003 Discharge of drilling wastes; discharge Barium 2 Increase in blood pressure from metal refineries; erosion 2 of natural deposits Anemia; decrease in blood Discharge from factories; leaching Benzene -

Scientific Committee on Consumer Safety SCCS
SCCS/1249/09 Revision of 28 September 2010 Scientific Committee on Consumer Safety SCCS OPINION ON Boron compounds The SCCS adopted this opinion at its 7th plenary meeting of 22 June 2010 SCCS/1249/09 Opinion on boron compounds ___________________________________________________________________________________________ About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Jürgen Angerer, Ulrike Bernauer, Claire Chambers, Qasim Chaudhry, Gisela Degen, Gerhard Eisenbrand, Thomas Platzek, Suresh Chandra Rastogi, Vera Rogiers, Christophe Rousselle, Tore Sanner, Kai Savolainen, Jacqueline Van Engelen, Maria Pilar Vinardell, Rosemary Waring, Ian R. -
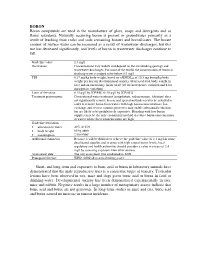
BORON Boron Compounds Are Used in the Manufacture of Glass, Soaps and Detergents and As Flame Retardants
BORON Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall. Guideline value 2.4 mg/l Occurrence Concentrations vary widely and depend on the surrounding geology and wastewater discharges. For most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l. TDI 0.17 mg/kg body weight, based on a BMDL 05 of 10.3 mg boron/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) Limit of detection 0.15 µg/l by ICP/MS; 6–10 µg/l by ICP/AES Treatment performance Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be installed in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. Guideline derivation • allocation to water 40% of TDI • body weight 60 kg adult • consumption 2 litres/day Additional comments Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. -

Silver Conductive Grease, Boron Nitride Heat Sink Grease, Silicone Free Heat Sink Grease
Silver Conductive Grease, Boron Nitride Heat Sink Grease, Silicone Free Heat Sink Grease Description CircuitWorks® Conductive Grease, otherwise known as thermal greases, thermal gel, thermal compound, thermal paste, heat paste, heat sink paste or heat sink compound, are conveniently packaged in syringes for fast and easy application, and to reduce mess and wastage. The range covers a number of electronic production, rework and repair applications. We offer three types of conductive grease: Silver Conductive Grease Boron Nitride Heat Sink Grease Silicone Free Heat Sink Grease Features & Benefits RoHS compliant Conveniently packaged to reduce waste and mess Silicone free option available compound will not harden or dry out Excellent thermal conductivity Silver Conductive Grease The CIR CW7100 CircuitWorks® syringe dispenser for precise application of grease to provide Silver Conductive Grease, Boron Nitride Heat Sink Grease, Silicone Free Heat Sink Grease superior electrical and thermal conductivity, lubrication and protection. Maximum electrical and thermal conductivity Protects against moisture and corrosion Thermally stable over a wide temperature range Conductive lubricant containing pure silver Fills connector gaps to maximize electrical and thermal conductivity Controls static discharge Grounds circuits Typical Applications Lubrication of substation switches or circuit breakers Heat dissipation from transformers Low or medium speed sliding contacts Static grounding on seals or O-rings Extending the life of rotating switches Specifications -

Effectiveness of Virginia's Water Resource Planning and Management
Commonwealth of Virginia House Document 8 (2017) October 2016 Report to the Governor and the General Assembly of Virginia Effectiveness of Virginia’s Water Resource Planning and Management 2016 JOINT LEGISLATIVE AUDIT AND REVIEW COMMISSION Joint Legislative Audit and Review Commission Chair Delegate Robert D. Orrock, Sr. Vice-Chair Senator Thomas K. Norment, Jr. Delegate David B. Albo Delegate M. Kirkland Cox Senator Emmett W. Hanger, Jr. Senator Janet D. Howell Delegate S. Chris Jones Delegate R. Steven Landes Delegate James P. Massie III Senator Ryan T. McDougle Delegate John M. O’Bannon III Delegate Kenneth R. Plum Senator Frank M. Ruff, Jr. Delegate Lionell Spruill, Sr. Martha S. Mavredes, Auditor of Public Accounts Director Hal E. Greer JLARC staff for this report Justin Brown, Senior Associate Director Jamie Bitz, Project Leader Susan Bond Joe McMahon Christine Wolfe Information graphics: Nathan Skreslet JLARC Report 486 ©2016 Joint Legislative Audit and Review Commission http://jlarc.virginia.gov February 8, 2017 The Honorable Robert D. Orrock Sr., Chair Joint Legislative Audit and Review Commission General Assembly Building Richmond, Virginia 23219 Dear Delegate Orrock: In 2015, the General Assembly directed the Joint Legislative Audit and Review Commission (JLARC) to study water resource planning and management in Virginia (HJR 623 and SJR 272). As part of this study, the report, Effectiveness of Virginia’s Water Resource Planning and Management, was briefed to the Commission and authorized for printing on October 11, 2016. On behalf of Commission staff, I would like to express appreciation for the cooperation and assistance of the staff of the Virginia Department of Environmental Quality and the Virginia Water Resource Research Center at Virginia Tech. -

Reference: Groundwater Quality and Groundwater Pollution
PUBLICATION 8084 FWQP REFERENCE SHEET 11.2 Reference: Groundwater Quality and Groundwater Pollution THOMAS HARTER is UC Cooperative Extension Hydrogeology Specialist, University of California, Davis, and Kearney Agricultural Center. roundwater quality comprises the physical, chemical, and biological qualities of UNIVERSITY OF G ground water. Temperature, turbidity, color, taste, and odor make up the list of physi- CALIFORNIA cal water quality parameters. Since most ground water is colorless, odorless, and Division of Agriculture without specific taste, we are typically most concerned with its chemical and biologi- and Natural Resources cal qualities. Although spring water or groundwater products are often sold as “pure,” http://anrcatalog.ucdavis.edu their water quality is different from that of pure water. In partnership with Naturally, ground water contains mineral ions. These ions slowly dissolve from soil particles, sediments, and rocks as the water travels along mineral surfaces in the pores or fractures of the unsaturated zone and the aquifer. They are referred to as dis- solved solids. Some dissolved solids may have originated in the precipitation water or river water that recharges the aquifer. A list of the dissolved solids in any water is long, but it can be divided into three groups: major constituents, minor constituents, and trace elements (Table 1). The http://www.nrcs.usda.gov total mass of dissolved constituents is referred to as the total dissolved solids (TDS) concentration. In water, all of the dissolved solids are either positively charged ions Farm Water (cations) or negatively charged ions (anions). The total negative charge of the anions always equals the total positive charge of the cations. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

Synthesis, Characterization and Fungicidal Effects of Some
Journal of Manmohan Memorial Institute of Health Sciences (JMMIHS) Synthesis, Characterization and Fungicidal Effects of some Organoborates Shaheen Akther1 Khanal DP2 Ullah SS3 Abstract Synthesis of benzoyl borate, phthasloyl borate and oxyl borate was done and characterized by infra red, ultraviolet and mass spectra. All newly synthesized and characterized organoborates were tested for their antifungal activity against pencillium that spoilt mostly celluloid materials stored in museum. All synthesized organoborates are unstable in room temperature and quickly degraded on exposure to air. Benzoyl borate found to be most active antifungal compound of the series against penicillium. The fungicidal activity increases in hydrophobicity and electron withdrawing nature of the benzene ring substituents. These compounds may be good fungicidal for the conservation of celluloid materials lying in the museum. Key words: Organoborates, Silver metaborate, Acyl chloride, spore, Antifungal. Introduction All organoborates are synthesized with the used of silver metaborate (AgBO2) and boric acid [1] with organic halides such as 3,5, dinitrobenzoyl chloride and p-nitrobenzoyl chloride. Since the silver atom in AgBO2 in photo activated state the reaction with organic halides having a polarized halogen atom will readily takes place with the formation of silver halides. Steric effect is an important factor in determining the course of reactions well as the stability of the product. It is observed that acyl chloride reacts more readily than sterically hindered pyrollium chloride [2]. The reaction of silver metaborate with organic halides may be ascribed to an ionic mechanism. Indeed acyllium [3] ion have been recognized as possible intermediate in acid catalyzed etherification in Friedel- Crafts reactions through the universality of such mechanism in doubt and the ion is assumed to resonate between the below structures: R – C O+ R – C+ O The presence of CO group polarizes the carbon halogen bond to great extent. -

A Public Health Legal Guide to Safe Drinking Water
A Public Health Legal Guide to Safe Drinking Water Prepared by Alisha Duggal, Shannon Frede, and Taylor Kasky, student attorneys in the Public Health Law Clinic at the University of Maryland Carey School of Law, under the supervision of Professors Kathleen Hoke and William Piermattei. Generous funding provided by the Partnership for Public Health Law, comprised of the American Public Health Association, Association of State and Territorial Health Officials, National Association of County & City Health Officials, and the National Association of Local Boards of Health August 2015 THE PROBLEM: DRINKING WATER CONTAMINATION Clean drinking water is essential to public health. Contaminated water is a grave health risk and, despite great progress over the past 40 years, continues to threaten U.S. communities’ health and quality of life. Our water resources still lack basic protections, making them vulnerable to pollution from fracking, farm runoff, industrial discharges and neglected water infrastructure. In the U.S., treatment and distribution of safe drinking water has all but eliminated diseases such as cholera, typhoid fever, dysentery and hepatitis A that continue to plague many parts of the world. However, despite these successes, an estimated 19.5 million Americans fall ill each year from drinking water contaminated with parasites, bacteria or viruses. In recent years, 40 percent of the nation’s community water systems violated the Safe Drinking Water Act at least once.1 Those violations ranged from failing to maintain proper paperwork to allowing carcinogens into tap water. Approximately 23 million people received drinking water from municipal systems that violated at least one health-based standard.2 In some cases, these violations can cause sickness quickly; in others, pollutants such as inorganic toxins and heavy metals can accumulate in the body for years or decades before contributing to serious health problems. -

WHO Guidelines for Indoor Air Quality : Selected Pollutants
WHO GUIDELINES FOR INDOOR AIR QUALITY WHO GUIDELINES FOR INDOOR AIR QUALITY: WHO GUIDELINES FOR INDOOR AIR QUALITY: This book presents WHO guidelines for the protection of pub- lic health from risks due to a number of chemicals commonly present in indoor air. The substances considered in this review, i.e. benzene, carbon monoxide, formaldehyde, naphthalene, nitrogen dioxide, polycyclic aromatic hydrocarbons (especially benzo[a]pyrene), radon, trichloroethylene and tetrachloroethyl- ene, have indoor sources, are known in respect of their hazard- ousness to health and are often found indoors in concentrations of health concern. The guidelines are targeted at public health professionals involved in preventing health risks of environmen- SELECTED CHEMICALS SELECTED tal exposures, as well as specialists and authorities involved in the design and use of buildings, indoor materials and products. POLLUTANTS They provide a scientific basis for legally enforceable standards. World Health Organization Regional Offi ce for Europe Scherfi gsvej 8, DK-2100 Copenhagen Ø, Denmark Tel.: +45 39 17 17 17. Fax: +45 39 17 18 18 E-mail: [email protected] Web site: www.euro.who.int WHO guidelines for indoor air quality: selected pollutants The WHO European Centre for Environment and Health, Bonn Office, WHO Regional Office for Europe coordinated the development of these WHO guidelines. Keywords AIR POLLUTION, INDOOR - prevention and control AIR POLLUTANTS - adverse effects ORGANIC CHEMICALS ENVIRONMENTAL EXPOSURE - adverse effects GUIDELINES ISBN 978 92 890 0213 4 Address requests for publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Scherfigsvej 8 DK-2100 Copenhagen Ø, Denmark Alternatively, complete an online request form for documentation, health information, or for per- mission to quote or translate, on the Regional Office web site (http://www.euro.who.int/pubrequest).