Borax by the Forest Service from 2000 to 2004
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Living up to Life Special Stain Kit Modified Grocott's Methenamine
Living up to Life Special Stain Kit Modified Grocott’s Methenamine Silver Stain Catalog No: 38016SS12 Intended Use 16. Rinse slides briefly in deionized water. For In Vitro Diagnostic Use. For Laboratory Use. 17. Dehydrate slides in three changes of absolute alcohol. The reagents in this kit are intended for “In Vitro“ use only. The Modified Grocott’s Methenamine 18. Clear slides in two changes of xylene and mount in a xylene miscible medium. Silver Stain when used with appropriate histological protocols may be used for the demonstration of defined fungi and infectious agents such as Aspergillus sp., Pneumocystis Staining Protocol (Microwave) carinii and Cryptococcus neoformans in formalin fixed, paraffin embedded tissue sections. Exercise caution when using the microwave oven to heat any solution or reagent. The microwave must be properly ventilated to prevent the accumulation of fumes in the laboratory. Probable Mode of Action Microwave transparent Coplin jars and caps should be used during the staining process. The The mechanism of action of Modified Grocott’s Methenamine Silver Stain is based upon the caps should be loosely attached to prevent spills. Caps with ventilation holes also may be used. capacity of aldehyde groups to reduce cationic silver (Ag+) to metallic silver. Chromic acid is All microwave ovens should be used in accordance with the manufacturer’s instructions. The used to generate aldehyde groups by the oxidation of 1-2 glycol groups within polysaccharide procedures described here were performed using an Energy Beam Sciences H2250 laboratory rich tissue components, e.g. glycogen, mucin, reticulin and fungal cell walls. When cationic microwave. -

Scientific Committee on Consumer Safety SCCS
SCCS/1249/09 Revision of 28 September 2010 Scientific Committee on Consumer Safety SCCS OPINION ON Boron compounds The SCCS adopted this opinion at its 7th plenary meeting of 22 June 2010 SCCS/1249/09 Opinion on boron compounds ___________________________________________________________________________________________ About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Jürgen Angerer, Ulrike Bernauer, Claire Chambers, Qasim Chaudhry, Gisela Degen, Gerhard Eisenbrand, Thomas Platzek, Suresh Chandra Rastogi, Vera Rogiers, Christophe Rousselle, Tore Sanner, Kai Savolainen, Jacqueline Van Engelen, Maria Pilar Vinardell, Rosemary Waring, Ian R. -
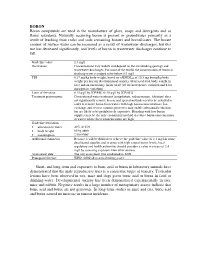
BORON Boron Compounds Are Used in the Manufacture of Glass, Soaps and Detergents and As Flame Retardants
BORON Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall. Guideline value 2.4 mg/l Occurrence Concentrations vary widely and depend on the surrounding geology and wastewater discharges. For most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l. TDI 0.17 mg/kg body weight, based on a BMDL 05 of 10.3 mg boron/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) Limit of detection 0.15 µg/l by ICP/MS; 6–10 µg/l by ICP/AES Treatment performance Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be installed in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. Guideline derivation • allocation to water 40% of TDI • body weight 60 kg adult • consumption 2 litres/day Additional comments Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. -

Synthesis, Characterization and Fungicidal Effects of Some
Journal of Manmohan Memorial Institute of Health Sciences (JMMIHS) Synthesis, Characterization and Fungicidal Effects of some Organoborates Shaheen Akther1 Khanal DP2 Ullah SS3 Abstract Synthesis of benzoyl borate, phthasloyl borate and oxyl borate was done and characterized by infra red, ultraviolet and mass spectra. All newly synthesized and characterized organoborates were tested for their antifungal activity against pencillium that spoilt mostly celluloid materials stored in museum. All synthesized organoborates are unstable in room temperature and quickly degraded on exposure to air. Benzoyl borate found to be most active antifungal compound of the series against penicillium. The fungicidal activity increases in hydrophobicity and electron withdrawing nature of the benzene ring substituents. These compounds may be good fungicidal for the conservation of celluloid materials lying in the museum. Key words: Organoborates, Silver metaborate, Acyl chloride, spore, Antifungal. Introduction All organoborates are synthesized with the used of silver metaborate (AgBO2) and boric acid [1] with organic halides such as 3,5, dinitrobenzoyl chloride and p-nitrobenzoyl chloride. Since the silver atom in AgBO2 in photo activated state the reaction with organic halides having a polarized halogen atom will readily takes place with the formation of silver halides. Steric effect is an important factor in determining the course of reactions well as the stability of the product. It is observed that acyl chloride reacts more readily than sterically hindered pyrollium chloride [2]. The reaction of silver metaborate with organic halides may be ascribed to an ionic mechanism. Indeed acyllium [3] ion have been recognized as possible intermediate in acid catalyzed etherification in Friedel- Crafts reactions through the universality of such mechanism in doubt and the ion is assumed to resonate between the below structures: R – C O+ R – C+ O The presence of CO group polarizes the carbon halogen bond to great extent. -

The Effects of Borax on Milk Yield and Selected Metabolic Parameters in Austrian Simmental (Fleckvieh) Cows
Veterinarni Medicina, 60, 2015 (4): 175–180 Original Paper doi: 10.17221/8104-VETMED The effects of borax on milk yield and selected metabolic parameters in Austrian Simmental (Fleckvieh) cows M. Kabu, C. Uyarlar Faculty of Veterinary Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey ABSTRACT: This study was conducted to determine the effects of orally administered borax on milk yield and on several blood variables related to metabolism in early lactation in Austrian Simmental cows (Fleckvieh). Twenty primiparous cows were selected at parturition and then assigned to one of two groups, the control group or the borax group. The study lasted for four weeks. Borax was administered orally at 0.2 mg/kg/day (Boron group) to all treatment cows shortly after the noon milking, whereas cows in the control group were not treated. All cows consumed the same diet. All feeds in the diet were analysed for crude cellulose, protein, ether extract, ash, and dry matter according to the Weende Analysis Systems, in addition to ADF and NDF, according to Van Soest. Blood samples were collected from all cows via the vena jugularis on lactation Days 0, 7, 15, 21 and 28 and ana- lysed for the following: serum boron (B), non-esterified fatty acids (NEFA), beta-hydroxybutyric acid (BHBA), total cholesterol (TChol), high density lipids (HDL), total protein (TP), blood urea nitrogen (BUN), albumin (ALB), creatine (CRE), uric acid (UA), glucose (GLU), aspartate aminotransferase (AST), alanine aminotrans- ferase (ALT) and gamma-glutamyl transpeptidase (GGT) concentrations. Serum B concentration was higher in the borax group than in the control group at Weeks 1, 2, 3, and 4 of the experiment. -

Technical Report: Barium Diboron Tetraoxide
Technical report: barium diboron tetraoxide Agency technical report on the classification and labelling of Barium diboron tetraoxide EC Number: 237-222-4 CAS Number: 13701-59-2 Health and Safety Executive Chemicals Regulation Division Redgrave Court Merton Road Bootle L20 7HS [email protected] Date: MAY 2021 1 Technical report: barium diboron tetraoxide Contents Brief summary ........................................................................................................................... 3 Introduction ............................................................................................................................... 4 Overview of current and proposed classification and labelling ................................................ 4 General substance information: ................................................................................................ 6 Background ................................................................................................................................ 6 Scientific assessment of the physical, human health and environmental hazard classes ........ 7 Physical Hazards ........................................................................................................................ 7 Health Hazards .......................................................................................................................... 7 Acute toxicity ......................................................................................................................... 7 Specific -

Pharmacokinetics of Pancreatic Polypeptide in Man
Gut: first published as 10.1136/gut.19.10.907 on 1 October 1978. Downloaded from Gut, 1978, 19, 907-909 Pharmacokinetics of pancreatic polypeptide in man T. E. ADRIAN, G. R. GREENBERG, H. S. BESTERMAN, AND S. R. BLOOM From the Department ofMedicine, Royal Postgraduate Medical School, Hammersmith Hospital, London SUMMARY Pure bovine pancreatic polypeptide (PP) was infused into 23 healthy subjects at doses of 1, 3, and 5 pmol/kg/min over 60 minutes and plasma PP was measured by radioimmunoassay. During the infusions mean plasma levels of 203 + 34, 575 + 73, and 930 ± 48 pmol/l respectively were achieved. Mean disappearance half time on stopping the infusion was 6-9 + 0 3 min (mean + SEM). The metabolic clearance rate was 5-1 + 0-2 ml/kg/min (mean + SEM) and the apparent volume of distribution was calculated to be 51 ± 3 ml/kg (mean ± SEM). This study provides for the first time pharmacokinetic data for PP in man. Pancreatic polypeptide (PP) is a recently discovered syringe ram pump for a basal period of 30 minutes. hormonal 36 amino acid peptide localised in an BPP was then added to the infusion at nominal endocrine cell of the mammalian and avian pancreas doses of 1 (n = 6), 3 (n = 9), and 5 (n = 8) pmol/ (Kimmel et al., 1968; Lin and Chance, 1972). PP cir- kg/min. culates in plasma and levels rise substantially on the Blood samples were collected in chilled tubes with ingestion of a meal and remain raised for several 10 units heparin and 400 KIU aprotinin (Trasylol) hours (Adrian et al., 1976; Floyd et al., 1976; per ml, centrifuged, and the plasma frozen within 15 Schwartz et al., 1976). -

(12) Patent Application Publication (10) Pub. No.: US 2017/0037303 A1 Waller Et Al
US 20170037303A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0037303 A1 Waller et al. (43) Pub. Date: Feb. 9, 2017 (54) COMPOSITIONS AND METHODS FOR E2IB 43/267 (2006.01) DELAYED CROSSLINKING IN HYDRAULC C09K 8/90 (2006.01) FRACTURING FLUIDS E2IB 37/06 (2006.01) (52) U.S. Cl. (71) Applicant: Ecolab USA Inc., St. Paul, MN (US) CPC ................. C09K 8/685 (2013.01); C09K 8/68 (2013.01); C09K 8/90 (2013.01); C09K 8/887 (72) Inventors: Christopher Waller, Houston, TX (2013.01); E2IB 37/06 (2013.01); E2IB 43/26 (US); Kirk E. Wells, Sugar Land, TX (2013.01); E2IB 43/267 (2013.01); E2IB (US); Brian Mueller, Missouri City, 33/138 (2013.01) TX (US); Trinh Tran, Manrel, TX (US); Pablo Munoz, Katy, TX (US) (57) ABSTRACT (21) Appl. No.: 15/225,879 Disclosed herein are compositions and methods for delaying (22) Filed: Aug. 2, 2016 crosslinking in injectable compositions for hydraulic frac turing and related applications. The compositions and meth Related U.S. Application Data ods are effective in injectable compositions comprising or Substantially excluding dissolved reactive species. The com (60) Provisional application No. 62/200,172, filed on Aug. positions and methods provide delayed crosslinking at high 3, 2015, provisional application No. 62/362.691, filed temperatures and pressures, such as those encountered by on Jul. 15, 2016. hydraulic fracturing compositions injected into Subterranean O O environments. Compositions include injectable solutions Publication Classification comprising a competing agent that is the reaction product of (51) Int. Cl. a dialdehyde having 2 to 4 carbon atoms with a non C09K 8/68 (2006.01) polymeric cis-hydroxyl compound. -

Available At
Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 VOL. IV.—1890. c THE JOURNAL OF LARYN GO LOGY AND RHINOLOGY; AN ANALYTICAL RECORD OF CURRENT LITERATURE RELATING TO THE THROAT AND NOSE. EDITED BY SIR MORELL MACKENZIE, M.D. Lond., AM) R. NORRIS WOLFENDEN, M.D. Cantab., WITH TUT'. CO-OI'EKAl IuN OF DR. JOHN N. MACKENZIE (BALTIMORE) DR. VALERIUS IDELSON (BERNE) DR. BRYSON DELAVAN (NEW YORK) DR. MICHAEL (HAMBURG) DR. W. PORTER (ST. LOUIS) PROF. RAMON DE LA SOTA Y LASTRA DR. JOAL (MONT DORE) (SEVILLE) PROF. MASSEI (NAPLES) DR. HOLGER MYGIND (COPENHAGEN) PROF. GUYE (AMSTERDAM) DR. SMYLY (DUBLIN) DR. CAPART (BRUSSELS) DR. DAVID COLLINGWOOD (SYDNEY; DR. CONSTANTIN KARWOWSKI (WARSAW) DR. G. W. MAJOR (MONTREAL) DR. BARCLAY J. BARON (BRISTOL) DR. MAXWELL ROSS (EDINBURGH) Dr. G. HUNTER MACKENZIE (Edinburgh). F. A. DAVIS, LONDON: PHILADELPHIA: 40, BERNERS STREET, \V. 1231, FILBERT STREET. Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.19, on 24 Sep 2021 at 18:25:48, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S1755145500148705 CONTENTS. -

The Influence of the Presence of Borax and Nacl on Water
sensors Letter The Influence of the Presence of Borax and NaCl on Water Absorption Pattern during Sturgeon Caviar (Acipenser transmontanus) Storage Massimo Brambilla 1 , Marina Buccheri 2, Maurizio Grassi 2 , Annamaria Stellari 1, Mario Pazzaglia 3, Elio Romano 1 and Tiziana M. P. Cattaneo 2,* 1 Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria–CREA–Centro di Ricerca Ingegneria e Trasformazioni Agroalimentari, Via Milano, 43–24047 Treviglio (Bergamo), Italy; [email protected] (M.B.); [email protected] (A.S.); [email protected] (E.R.) 2 Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria–CREA–Centro di Ricerca Ingegneria e Trasformazioni Agroalimentari, Via G. Venezian, 26–20133 Milano, Italy; [email protected] (M.B.); [email protected] (M.G.) 3 Agroittica Lombarda S.p.A., Via Kennedy, 25012 Calvisano Brescia, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-02-239-557-212 Received: 10 November 2020; Accepted: 10 December 2020; Published: 15 December 2020 Abstract: Sturgeon caviar quality relies not only on the perfect dosage of the ingredients but also on the long sturgeon breeding cycle (about 12–15 years) and the exact timing of the egg extraction. For the improvement and the promotion of Italian caviar, the development of an analytical system dedicated to fish products, and caviar, in particular, is fundamental. The use of near-infrared spectrometry (NIRS) technology is auspicious. The aquaphotomics approach proved to be an adequate analytical tool to highlight, in real-time, the differences in caviar quality stored with, or without, borax as a preservative. -

Laboratory Evaluation of Borate:Amine:Copper Derivatives In
LABORATORY EVALUATION OF BORATE:AMINE:COPPER DERIVATIVES IN WOOD FOR FUNGAL DECAY PROTECTION George Chen Research Chemist Performance Enhanced Biopolymers USDA Forest Service, Forest Products Laboratory 1 Gifford Pinchot Drive Madison, WI 53726 (Received December 2010) Abstract. This study aimed to evaluate borate:amine:copper derivatives in wood for fungal decay protection as well as the permanence of copper and boron in wood. Each of four derivatives of borate: amine:copper prevented fungal decay in wood. Disodium tetraborate decahydrate (borax):amine:copper derivatives with 0.61-0.63% retention after water leaching prevented decay by Gloeophyllum trabeum (Gt) and with 0.64% retention prevented decay by Trametes versicolor (Tv). Leaching did not decrease decay resistance for either Gt or Tv. Disodium octaborate tetrahydrate (DOT):amine:copper derivatives with 1.14-2.93% retention after water leaching prevented decay by Gt and with 0.54-1.19% retention prevented decay by Tv. Leaching decreased decay resistance to Gt but not to Tv. Higher copper and boron in disodium borax:amine:copper derivatives contributed to more decay resistance to Gt and Tv than that of DOT:amine:copper derivatives as evidenced by elemental analysis. IR spectra of wood treated with 5% borate:amine:copper derivatives after water leaching showed increased absorption at 1632-1635 cmÀ1 compared with the control. This increased absorption was partly attributable to carbonyl of copper carboxylates from oxidation of hemiacetals of hemicelluloses and cellulose by copper (II) ions and carbonyls of copper (II) quinone methides by oxidation of guaicyls by copper (II) ions. It was also partly attributable to carbonyls of copper carboxylates from hemicelluloses and phenolates from lignin through ion exchange reactions. -

Volume 42, Number 4, 2010
BULGARIAN CHEMICAL COMMUNICATIONS 2010 Volume 42 / Number 4 Journal of the Chemical Institutes of the Bulgarian Academy of Sciences and of the Union of Chemists in Bulgaria Announcement Seventh National Conference on Chemistry and 2nd International Conference on Green Technologies and Environmental Protection INVITATION 2. Organic chemistry: V. Dimitrov, P. K. The Union of Chemists in Bulgaria (UCB) Sharma extends a cordial invitation to all scientists 3. Inorganic chemistry: D. Todorovsky, R. in the field of chemistry to participate in the K. Sharma Seventh National Conference on Chemistry 4. Analytical chemistry: D. Tsalev, R. K. to be held at the University of Chemical Mahajan Technology and Metallurgy in Sofia 5. Catalysis: S. Damyanova, R. D. Kaushik (Bulgaria) from 26 to 29 May 2011. 6. Chemical engineering: V. Beshkov, M. Parallely the 2nd International Conference A. Abdullaha on Green Technologies and Environmental 7. Chemistry and environmental protection: Protection co-organized with the Chaudhary Y. Pelovsky, A. Mittal Charan Singh University, Meerut (India) 8. Polymers (plastics, rubbers, chemical and Devam Foundation – Bulgarian-Indian fibres, cellulose): N. Dishovsky, R. K. Soni Art & Culture Centre will take place. 9. Chemical technologies: G. Vissokov, A. Muddoo ORGANIZATION INSTITUTIONS 10. Oil processing, petrochemistry and Union of Chemists in Bulgaria, Union of organic synthesis: G. Cholakov, A. P. Gupta Scientists in Bulgaria, Bulgarian Academy 11. Chemical education: B. Toshev, A. K. of Sciences, Chaudhary Charan Singh Halve University, Meerut (India), Devam Foundation, University of Chemical GENERAL SCOPE Technology and Metallurgy, Sofia, Prof. A. The Seventh National Conference on Zlatarov University of Burgas, Faculty of Chemistry is organized within the Chemistry at St.