MATERIALS DATA HANDBOOK Aluminum Alloy 5456 (2Nd Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -
Linear) Thermal Expansion for Selected Materials (COE Or CTE
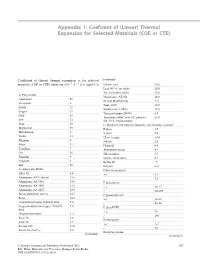
Appendix 1: Coefcient of (Linear) Thermal Expansion for Selected Materials (COE or CTE) Coefficient of (linear) thermal expansion, α, for selected (continued) − − materials (COE or CTE) (units are ×10 6 °C 1 (i.e. ppm/°C)) Indium–lead 33.0 Lead (95 %) tin solder 28.0 Tin–lead solder 60/40 25.0 A. Pure metals Magnesium, AZ31B 26.0 Aluminium 25 Ni-clad Molybdenum 5–6 Chromium 6 Steel, 1020 12.0 Cobalt 12 Stainless steel (18-8) 17.0 Copper 17 Tungsten/copper (90/10) 6.5 Gold 14 Aluminium MMC with SiC particles 6–14 Iron 12 (80–50 % reinforcement) Lead 29 C. Insulators and substrate materials (for electronic systems)a Magnesium 25 E glass 5.5 Molybdenum 5 S glass 2.6 Nickel 13 Glass–ceramic >3.0 Platinum 9 Silicon 2.6 Silver 19 Diamond 0.9 Tantalum 7 Aluminium nitride 4.5 Tin 20 Silicon nitride 3.7 Titanium 9 Quartz, fused silica 0.5 Tungsten 5 Kevlar 49 –5 Zinc 35 Beryllia 6–9 B. Alloys and MMCs Cubic boron nitride Alloy 42 4.4 x–y 3.7 Aluminium (40 % silicon) 13.5 z 7.2 Aluminium, AA 6061 23.6 E glass/epoxy Aluminium, AA 3003 23.2 x–y 14–17 Aluminium, AA 2017 22.9 z 80–280 Boron aluminium (20 %) 12.7 E glass/polyimide Brass 18.0 x–y 12–16 Copper/invar/copper 20/60/20 thick 5.8 z 40–80 Copper/molybdenum/copper 20/60/20 7.0 E glass/PTFE thick x–y 24 Graphite/aluminium 4–6 z 260 Invar 36 1.6 Kevlar/epoxy Invar 42 4.5 x–y 5–7 Inconel 600 13.0 z 70 Kovar (Fe–Ni–Co) 5.0 (continued) Kevlar/polyimide (continued) © Springer International Publishing Switzerland 2016 557 B.D. -

924 Iaea-Sm-310/ 69P
924 IAEA-SM-310/ 69P RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN M.I. de Vries Netherlands Energy Research Foundation, ECN, Eetten, The Netherlands M.R.. Cundy Joint Research Centre, Patten, The Netherlands 925 RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN ABSTRACT Results are reported from hardness measurements, tensile tests and fracture mechanics experiments (fatigue crack growth and fracture toughness) on 5154 aluminium specimens, fabricated from remnants of the old HFR core box. The specimen material was exposed to a maximum thermal neutron fluence of 7.5 * 1026n/m2(E < 0.4eV). Test results for this fluence (ratio of the thermal to fast neutron flux density is 1.17) are: hardness 63HR15N, 0.2 - yield strength 525 MPa and total elongation 2.2% strain. Material which was exposed to a lower thermal fluence of 5.6 * 10 n/m , but with a thermal to fast neutron ratio of about 4, shows more radiation hardening : 67HR15N, 0.2 - yield strength 580 MPa and 1.5% total elongation. -5 -3 Fatigue crack growth rates range from 5 * 10 mm/cycle to 10 mm/cycle for AK ranging from 8 to 20 MPa)|m. The most highly exposed (7.5 * 10 n/m ) material shows accelerated fatigue crack growth due to unstable crack extension at AK of about 15 Mpa^m. The lowermost meaningful measure of plane strain fracture toughness is 18 MPa^m. Except for the fracture toughness which is a factor of about 3 higher the results show reasonable agreement with the expected mechanical properties estimated in the "safe end-of-life" assessment of the old HFR vessel. -

Machining of Aluminum and Aluminum Alloys / 763
ASM Handbook, Volume 16: Machining Copyright © 1989 ASM International® ASM Handbook Committee, p 761-804 All rights reserved. DOI: 10.1361/asmhba0002184 www.asminternational.org MachJning of Aluminum and AlumJnum Alloys ALUMINUM ALLOYS can be ma- -r.. _ . lul Tools with small rake angles can normally chined rapidly and economically. Because be used with little danger of burring the part ," ,' ,,'7.,','_ ' , '~: £,~ " ~ ! f / "' " of their complex metallurgical structure, or of developing buildup on the cutting their machining characteristics are superior ,, A edges of tools. Alloys having silicon as the to those of pure aluminum. major alloying element require tools with The microconstituents present in alumi- larger rake angles, and they are more eco- num alloys have important effects on ma- nomically machined at lower speeds and chining characteristics. Nonabrasive con- feeds. stituents have a beneficial effect, and ,o IIR Wrought Alloys. Most wrought alumi- insoluble abrasive constituents exert a det- num alloys have excellent machining char- rimental effect on tool life and surface qual- acteristics; several are well suited to multi- ity. Constituents that are insoluble but soft B pie-operation machining. A thorough and nonabrasive are beneficial because they e,,{' , understanding of tool designs and machin- assist in chip breakage; such constituents s,~ ,.t ing practices is essential for full utilization are purposely added in formulating high- of the free-machining qualities of aluminum strength free-cutting alloys for processing in alloys. high-speed automatic bar and chucking ma- Strain-hardenable alloys (including chines. " ~ ~p /"~ commercially pure aluminum) contain no In general, the softer ailoys~and, to a alloying elements that would render them lesser extent, some of the harder al- c • o c hardenable by solution heat treatment and ,p loys--are likely to form a built-up edge on precipitation, but they can be strengthened the cutting lip of the tool. -

Aluminium Alloys Chemical Composition Pdf
Aluminium alloys chemical composition pdf Continue Alloy in which aluminum is the predominant lye frame of aluminum welded aluminium alloy, manufactured in 1990. Aluminum alloys (or aluminium alloys; see spelling differences) are alloys in which aluminium (Al) is the predominant metal. Typical alloy elements are copper, magnesium, manganese, silicon, tin and zinc. There are two main classifications, namely casting alloys and forged alloys, both further subdivided into heat-treatable and heat-free categories. Approximately 85% of aluminium is used for forged products, e.g. laminated plates, foils and extrusions. Aluminum cast alloys produce cost-effective products due to their low melting point, although they generally have lower tensile strength than forged alloys. The most important cast aluminium alloy system is Al–Si, where high silicon levels (4.0–13%) contributes to giving good casting features. Aluminum alloys are widely used in engineering structures and components where a low weight or corrosion resistance is required. [1] Alloys composed mostly of aluminium have been very important in aerospace production since the introduction of metal leather aircraft. Aluminum-magnesium alloys are both lighter than other aluminium alloys and much less flammable than other alloys containing a very high percentage of magnesium. [2] Aluminum alloy surfaces will develop a white layer, protective of aluminum oxide, if not protected by proper anodization and/or dyeing procedures. In a wet environment, galvanic corrosion can occur when an aluminum alloy is placed in electrical contact with other metals with a more positive corrosion potential than aluminum, and an electrolyte is present that allows the exchange of ions. -

Aluminum Alloy Weldability: Identification of Weld Solidification Cracking Mechanisms Through Novel Experimental Technique and Model Development
Dipl.-Ing. Nicolas Coniglio Aluminum Alloy Weldability: Identifi cation of Weld Solidifi cation Cracking Mechanisms through Novel Experimental Technique and Model Development BAM-Dissertationsreihe • Band 40 Berlin 2008 Die vorliegende Arbeit entstand an der BAM Bundesanstalt für Materialforschung und -prüfung. Impressum Aluminum Alloy Weldability: Identifi cation of Weld Solidifi cation Cracking Mechanisms through Novel Experimental Technique and Model Development 2008 Herausgeber: BAM Bundesanstalt für Materialforschung und -prüfung Unter den Eichen 87 12205 Berlin Telefon: +49 30 8104-0 Telefax: +49 30 8112029 E-Mail: [email protected] Internet: www.bam.de Copyright © 2008 by BAM Bundesanstalt für Materialforschung und -prüfung Layout: BAM-Arbeitsgruppe Z.64 ISSN 1613-4249 ISBN 978-3-9812354-3-2 Aluminum Alloy Weldability: Identification of Weld Solidification Cracking Mechanisms through Novel Experimental Technique and Model Development Dissertation zur Erlangung des akademischen Grades Doktor-Ingenieur (Dr.-Ing.) genehmigt durch die Fakultät für Maschinenbau der Otto-von-Guericke-Universität Madgeburg am 02.06.08 vorgelegte Dissertation von Dipl.-Ing. Nicolas Coniglio Thesis Committee: Prof. Dr.-Ing. A. Bertram Prof. Dr.-Ing. T. Böllinghaus Prof. C.E. Cross Prof. S. Marya Date of Examination: 23 October 2008 Abstract Abstract The objective of the present thesis is to make advancements in understanding solidification crack formation in aluminum welds, by investigating in particular the aluminum 6060/4043 system. Alloy 6060 is typical of a family of Al-Mg-Si extrusion alloys, which are considered weldable only when using an appropriate filler alloy such as 4043 (Al-5Si). The effect of 4043 filler dilution (i.e. weld metal silicon content) on cracking sensitivity and solidification path of Alloy 6060 welds are investigated. -

International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys
International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys 1525 Wilson Boulevard, Arlington, VA 22209 www.aluminum.org With Support for On-line Access From: Aluminum Extruders Council Australian Aluminium Council Ltd. European Aluminium Association Japan Aluminium Association Alro S.A, R omania Revised: January 2015 Supersedes: February 2009 © Copyright 2015, The Aluminum Association, Inc. Unauthorized reproduction and sale by photocopy or any other method is illegal . Use of the Information The Aluminum Association has used its best efforts in compiling the information contained in this publication. Although the Association believes that its compilation procedures are reliable, it does not warrant, either expressly or impliedly, the accuracy or completeness of this information. The Aluminum Association assumes no responsibility or liability for the use of the information herein. All Aluminum Association published standards, data, specifications and other material are reviewed at least every five years and revised, reaffirmed or withdrawn. Users are advised to contact The Aluminum Association to ascertain whether the information in this publication has been superseded in the interim between publication and proposed use. CONTENTS Page FOREWORD ........................................................................................................... i SIGNATORIES TO THE DECLARATION OF ACCORD ..................................... ii-iii REGISTERED DESIGNATIONS AND CHEMICAL COMPOSITION -

Advantages of Aluminium
Aluminium Information Advantages of Aluminium Advantages of Aluminium A unique combination of properties makes aluminium and its alloys one of the most versatile engineering and construction materials available today. Lightweight Aluminium is one of the lightest available commercial metals with a density approximately one third that of steel or copper. Its high strength to weight ratio makes it particularly important to transportation industries allowing increased payloads and fuel savings. Catamaran ferries, petroleum tankers and aircraft are good examples of aluminium’s use in transport. In other fabrications, aluminium’s lightweight can reduce the need for special handling or lifting equipment. Excellent Corrosion Resistance Aluminium has excellent resistance to corrosion due to the thin layer of aluminium oxide that forms on the surface of aluminium when it is exposed to air. In many applications, aluminium can be left in the mill finished condition. Should additional protection or decorative finishes be required, then aluminium can be either anodised or painted. Strong Although tensile strength of pure aluminium is not high, mechanical properties can be markedly increased by the addition of alloying elements and tempering. You can choose the alloy with the most suitable characteristics for your application. Typical alloying elements are manganese, silicon, copper and magnesium. Strong at Low Temperatures Where as steel becomes brittle at low temperatures, aluminium increases in tensile strength and retains excellent toughness. 1 © Capral Aluminium Limited Aluminium Information Advantages of Aluminium Easy to Work Aluminium can be easily fabricated into various forms such as foil, sheets, geometric shapes, rod, tube and wire. It also displays excellent machinability and plasticity ideal for bending, cutting, spinning, roll forming, hammering, forging and drawing. -

Aluminium Sheet, Coil, Plate, Treadplate & Extrusions
Aluminium Sheet, Coil, Plate, Treadplate & Extrusions & & Treadplate , luminium Sheet, Coil, Plate Coil, Sheet, luminium A Extrusions 8 8 Photography courtesy of Alcoa Aluminium and INCAT. www.atlassteels.com.au Atlas Steels – Product Reference Manual Section 8 – Aluminium Sheet, Coil, Plate, Treadplate & Extrusions Aluminium Advantages of using Aluminium 1 Light weight – approximately /3 the density of steel. Strength – some alloys can be substantially strengthened by work or by heat treatment. Workability – easy formability, machinability and readily welded. Corrosion resistance – varies depending on the alloy; the best resist marine exposure. Non-toxic – often used in contact with food. Non-magnetic and non-sparking. Electrical conductivity – very high; sometimes used for electrical conductors. Thermal conductivity – high. Reflectivity – bright finish options available. Specifications Flat rolled aluminium alloy products stocked by Atlas generally comply with ASTM B209M or ASTM B928M with dimensions in ANSI H35.2. There is very close agreement between these standards and Australian/New Zealand standard AS/NZS 1734 and again close agreement with European standard EN 573. Aluminium Association publication “Aluminium standards and data” gives a very accessible summary of both specified data and useful information. Aluminium products are often cited as compliant with “AA specs” based on this. Treadplate is specified in ASTM B632M but this does not cover the product well. Most mills produce to their own specifications and particularly -

Coniglio Thesis
Dipl.-Ing. Nicolas Coniglio Aluminum Alloy Weldability: Identifi cation of Weld Solidifi cation Cracking Mechanisms through Novel Experimental Technique and Model Development BAM-Dissertationsreihe • Band 40 Berlin 2008 Die vorliegende Arbeit entstand an der BAM Bundesanstalt für Materialforschung und -prüfung. Impressum Aluminum Alloy Weldability: Identifi cation of Weld Solidifi cation Cracking Mechanisms through Novel Experimental Technique and Model Development 2008 Herausgeber: BAM Bundesanstalt für Materialforschung und -prüfung Unter den Eichen 87 12205 Berlin Telefon: +49 30 8104-0 Telefax: +49 30 8112029 E-Mail: [email protected] Internet: www.bam.de Copyright © 2008 by BAM Bundesanstalt für Materialforschung und -prüfung Layout: BAM-Arbeitsgruppe Z.64 ISSN 1613-4249 ISBN 978-3-9812354-3-2 Aluminum Alloy Weldability: Identification of Weld Solidification Cracking Mechanisms through Novel Experimental Technique and Model Development Dissertation zur Erlangung des akademischen Grades Doktor-Ingenieur (Dr.-Ing.) genehmigt durch die Fakultät für Maschinenbau der Otto-von-Guericke-Universität Madgeburg am 02.06.08 vorgelegte Dissertation von Dipl.-Ing. Nicolas Coniglio Thesis Committee: Prof. Dr.-Ing. A. Bertram Prof. Dr.-Ing. T. Böllinghaus Prof. C.E. Cross Prof. S. Marya Date of Examination: 23 October 2008 Abstract Abstract The objective of the present thesis is to make advancements in understanding solidification crack formation in aluminum welds, by investigating in particular the aluminum 6060/4043 system. Alloy 6060 is typical of a family of Al-Mg-Si extrusion alloys, which are considered weldable only when using an appropriate filler alloy such as 4043 (Al-5Si). The effect of 4043 filler dilution (i.e. weld metal silicon content) on cracking sensitivity and solidification path of Alloy 6060 welds are investigated. -

Aluminium Alloy 5454 - 'O' and H111 Sheet
Aluminium Alloy 5454 - 'O' and H111 Sheet SPECIFICATIONS TEMPER TYPES The most common tempers for 5454 aluminium are Commercial 5454 shown below EN 5454 • O - Soft • H111 - Some work hardening imparted by shaping Aluminium alloy 5454 has very good corrosionprocesses but less than required for H11 temper resistance, in particular to seawater and general• H22 - Work hardened by rolling then annealed to environmental conditions. Strength is medium to high and similar alloy 5754 with good strength in the quarter hard temperature range 65 to 170 degrees centigrade. It • H32 - Work hardened by rolling then stabilised by has a high fatigue strength. It is not suitable for low-temperature heat treatment to quarter hard complex or fine extrusions. Applications 5454 is typically used in: SUPPLIED FORMS ~ Road Transport Body-Building Alloy 5454 is supplied in most forms but is not suitable ~ Cemical and Process Plant for fine or complex extrusions: ~ Pressure Vessels, Containers, Boilers ~ Cryogenics • Plate ~ Marine & Off-shore incl. Masts, • Sheet ~ Pylons, poles & masts • Bar • Treadplate/Patterened Sheet CHEMICAL COMPOSITION • Tube BS EN 573-3:2009 • Wire Alloy 5454 Element % Present GENERIC PHYSICAL PROPERTIES Magnesium (Mg) 2.40 - 3.00 Property Value Manganese (Mn) 0.50 - 1.00 Density 2.69 g/cm³ Iron (Fe) 0.0 - 0.40 Melting Point 645 °C Zinc (Zn) 0.0 - 0.25 Thermal Expansion 23.6 x10-6 /K Silicon (Si) 0.0 - 0.25 Modulus of Elasticity 70.5 GPa Chromium (Cr) 0.05 - 0.20 Thermal Conductivity 135 W/m.K Titanium (Ti) 0.0 - 0.20 Electrical Resistivity -

CUMULATIVE INDEX September 1952 Through December 2014
Materials Park, Ohio 44073-0002 :: 440.338.5151 :: Fax 440.338.8542 :: www.asminternational.org :: [email protected] Published by ASM International® :: Data shown are typical, not to be used for specification or final design. CUMULATIVE INDEX September 1952 through December 2014 Alphabetical listing by tradename or other designation and code number Alloy Steel Aluminum Beryllium Bismuth Carbon Steel Cast Iron Ceramic Chromium Cobalt Copper Gold Iron Lead Magnesium Molybdenum Nickel Neodymium Plastic Silver Stainless Steel Tin Titanium Tool Steel Tungsten Zinc Contents Alphabetical Index by Material Name Pages 3 through 33 Alphabetical Index within Material Group Pages 37 through 67 Copyright © 2015, ASM International®. All rights reserved. DATA ON WORLD WIDE METALS AND ALLOYS Published by ASM International® Materials Park, Ohio 44073-0002 [email protected] 440-338-5151, Fax 440-338-8542 www.asminternational.org Copyright © 2015 ASM International® All rights reserved No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the written permission of the copyright owner. Great care is taken in the compilation and production of this publication, but it should be made clear that NO WARRANTIES, EXPRESS OR IMPLIED, INCLUDING, WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, ARE GIVEN IN CONNECTION WITH THIS PUBLICATION. Although this information is believed to be accurate by ASM, ASM cannot guarantee that favorable results will be obtained from the use of this publication alone. This publication is intended for use by persons having technical skill, at their sole discretion and risk.