CORROSION-OF-ALUMINIUM.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Connor Final Highlighted
Preliminary Development of a PPAM Actuated Pediatric Prosthetic Ankle Connor McNamara-Spackman A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science (Bioengineering) at the University of Otago, Dunedin, New Zealand. February 2021 Abstract The purpose of this research was to develop a preliminary design of a powered pediatric prosthetic ankle. Previous research identified the health risk of improper gait cycle and the lack of powered prosthetic ankle options for children. Costs for powered prosthetic ankles are too high (upwards of $5000 NZD), the sizes are too large and the weight is too significant for a child to benefit from. Current technologies for ankle joint actuation and materials for the prosthetic structure were evaluated and a conclusion of utilizing PPAMs was chosen due to their ability to generate the required 300 N of contraction force. CAD was used to model the structure of a prosthetic ankle and evaluate the FOS of the different material combinations while under static loading and fatigue simulations. HDPE and UHMWPE failed to withstand the simulations, while the aluminium alloy and stainless steel showed minimal faults from the simulations. MatLab was used to simulate the desired PPAM dimensions of 100 mm to determine the contraction force and contraction percentage that can be generated by the PPAM. The smallest PPAM found in research was 110 mm and showed promising results from their mathematical modeling. The overall height of the prosthetic was no greater than 110 mm and the membrane length of the PPAM was no greater than 100 mm, while successfully producing more than 300 N during contraction. -

Alcotec Aluminum Technical Guide
AlcoTec Aluminum Technical Guide Contents AlcoTec Aluminum Wire & Equipment Technical Guide Table of Contents AlcoTec Aluminum Wire & Equipment Technical Guide ......................................................................................................... 1 Table of Contents ................................................................................................................................................................ 1 Environmental Health and Safety ......................................................................................................................................... 3 Technical Services Heat Treatable & Non-Heat Treatable Base & Fillers ............................................................................................................. 6 Filler Alloys: Chemical Composition Limits & Physical Properties ......................................................................................... 7 Conversion Factors ............................................................................................................................................................ 7 Welded Joint Strength ......................................................................................................................................................... 8 Typical Tensile Properties - Groove Welds ............................................................................................................................ 9 Weld Profiles ..................................................................................................................................................................... -

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -
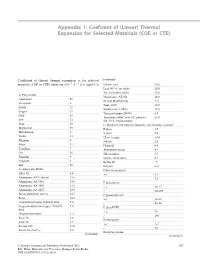
Linear) Thermal Expansion for Selected Materials (COE Or CTE
Appendix 1: Coefcient of (Linear) Thermal Expansion for Selected Materials (COE or CTE) Coefficient of (linear) thermal expansion, α, for selected (continued) − − materials (COE or CTE) (units are ×10 6 °C 1 (i.e. ppm/°C)) Indium–lead 33.0 Lead (95 %) tin solder 28.0 Tin–lead solder 60/40 25.0 A. Pure metals Magnesium, AZ31B 26.0 Aluminium 25 Ni-clad Molybdenum 5–6 Chromium 6 Steel, 1020 12.0 Cobalt 12 Stainless steel (18-8) 17.0 Copper 17 Tungsten/copper (90/10) 6.5 Gold 14 Aluminium MMC with SiC particles 6–14 Iron 12 (80–50 % reinforcement) Lead 29 C. Insulators and substrate materials (for electronic systems)a Magnesium 25 E glass 5.5 Molybdenum 5 S glass 2.6 Nickel 13 Glass–ceramic >3.0 Platinum 9 Silicon 2.6 Silver 19 Diamond 0.9 Tantalum 7 Aluminium nitride 4.5 Tin 20 Silicon nitride 3.7 Titanium 9 Quartz, fused silica 0.5 Tungsten 5 Kevlar 49 –5 Zinc 35 Beryllia 6–9 B. Alloys and MMCs Cubic boron nitride Alloy 42 4.4 x–y 3.7 Aluminium (40 % silicon) 13.5 z 7.2 Aluminium, AA 6061 23.6 E glass/epoxy Aluminium, AA 3003 23.2 x–y 14–17 Aluminium, AA 2017 22.9 z 80–280 Boron aluminium (20 %) 12.7 E glass/polyimide Brass 18.0 x–y 12–16 Copper/invar/copper 20/60/20 thick 5.8 z 40–80 Copper/molybdenum/copper 20/60/20 7.0 E glass/PTFE thick x–y 24 Graphite/aluminium 4–6 z 260 Invar 36 1.6 Kevlar/epoxy Invar 42 4.5 x–y 5–7 Inconel 600 13.0 z 70 Kovar (Fe–Ni–Co) 5.0 (continued) Kevlar/polyimide (continued) © Springer International Publishing Switzerland 2016 557 B.D. -

Tribological Behaviours Influenced by Surface Coatings and Morphology
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 7-11-2015 Tribological Behaviours Influenced yb Surface Coatings and Morphology Guang Wang University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Wang, Guang, "Tribological Behaviours Influenced yb Surface Coatings and Morphology" (2015). Electronic Theses and Dissertations. 5305. https://scholar.uwindsor.ca/etd/5305 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang A thesis Submitted to the Faculty of Graduate Studies through the Department of Mechanical, Automotive & Materials Engineering in Partial Fulfillment of the Requirements for the Degree of Master of Applied Science at the University of Windsor Windsor, Ontario, Canada 2015 ©2015 Guang Wang Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang APPROVED BY: Dr. -

Aluminum Sheet / Plate
Advanced Metal Technology Co.,Limited ALUMINUM PRODUCTS SERIES Advanced Metal Technology Co.,Limited 1 Hongnan Mansion, No.939 Jinqiao Road, Shanghai, China Post Code:200136 Tel: 0086-21-61623132 Fax: 0086-21-61622559 Henghua Technology Park,NAO.58dXiuvxi Raoand, WcuXei,Chdina Metal Technology Co.,Limited Post Code:214000 Tel: 0086-510-85192612 Fax: 0086-510-85192613 Flat/RM 1205, Tai Sang Bank Building, 130-132 Des Voeux Road Central, HK, China Advanced Metal Technology Co.,Limited CONTENT INTRODUCTION...............................................................................................................................3 ALUMINUM SHEET / PLATE..........................................................................................................3 1000 Series 1050 1060 1070 1100..............................................................................................6 5000 Series 5050 5054 5083 5454..............................................................................................7 6000 Series 6010 6011 6061 6062 6063.....................................................................................8 7000 Series 7005 7A04 7A09 7050 7075...................................................................................9 Aluminum Composite Sheet..................................................................................................... 10 Roof Ceiling.............................................................................................................................. 12 Air Plane Plate...........................................................................................................................14 -

Statistical Analysis of the Optical Interferometry of Pitting Process in Aluminum 3003 Sheets Exposed to Saline Environment
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Available online at www.sciencedirect.com ScienceDirect Procedia Materials Science 8 ( 2015 ) 82 – 90 International Congress of Science and Technology of Metallurgy and Materials, SAM - CONAMET 2013 Statistical Analysis of the Optical Interferometry of Pitting Process in Aluminum 3003 Sheets Exposed to Saline Environment Solange Y. Paredes-Dugarte, Benjamín Hidalgo-Prada Material Science Department, Materials Characterization Laboratory. Research Institute of Biomedicine and Applied Sciences “Dra. Susan Tai”, University Ave., Cumaná, Sucre 6101, Venezuela. Abstract In this study, a statistical evaluation was made of the susceptibility to pitting corrosion, using the pitting factor criteria. Specimens were cut in size of 15 cm x10 cm of AA3003 aluminium sheet of temper H14, H16 and H18 of national production. Afterwards, they were exposed to a salt spray during 72, 144, 216, 288 and 360 hours continually, according to ASTM B117 standard. After salt spray test was observed pitting attack in all specimens regardless of the exposure time and the degree of deformation (temper) of material. The surfaces of the corroded specimens were analyzed by optical interferometry. The parameters evaluated in each field were: roughness (Rms), peak-valley distance (PV) and the lowest point of all peaks (V). A pitting factor of the order of 4 was calculated, indicating a highly localized corrosion process for this commercial aluminium alloy 3003 in saline environment. © 20152014 TheThe Authors. Authors. Published Published by Elsevierby Elsevier Ltd. LtdThis. is an open access article under the CC BY-NC-ND license Selection(http://creativecommons.org/licenses/by-nc-nd/4.0/ and peer-review under responsibility). -

Abstracts from the Scientific and Technical Press Titles And
December, ig4j Abstracts from the Scientific and Technical Press " (No. 117. October, 1943) AND Titles and References of Articles and Papers Selected from Publications (Reviewed by R.T.P.3) TOGETHER WITH List of Selected Translations (No. 63)' London : "THE ROYAL AERONAUTICAL SOCIETY" with which is incorporated "The Institution of Aeronautical Engineers" 4, Hamilton Place, W.I Telephone: Grosvenor 3515 (3 lines) ABSTRACTS FROM THE SCIENTIFIC AND TECHNICAL PRESS. Issued by the Directorate's of Scientific Research and Technical Development, Ministry of Air craft Production. (Prepared by R.T.P.3.) No. 117. OCTOBER, 1943. Notices and abstracts from the Scientific and Technical Press are prepared primarily for_ the information of Scientific and Technical Staffs. Particular attention is paid to the work carried out in foreign countries, on the assumption that the more accessible British work (for example that published by the Aeronautical Research Committee^ is already known to these Staffs. Requests from scientific and technical staffs for further information of transla tions should be addressed to R.T,P.3, Ministry of Aircraft Production, and not to the Royal Aeronautical Society. Only a limited number of the articles quoted from foreign journals are trans lated and usually only the original can be supplied on loan. If, however, translation is required, application should be made in writing to R.T.P.3, the requests being considered in accordance with existing facilities. ' NOTE.—As far as possible, the country of origin quoted in the items refers to the original source. The Effect of Nitrogen on the Properties of Certain Austenitic Valve Steels. -

Lead Action News Lanv15n2 Combating the Silent Epidemic
LEAD Action News vol. 15 no. 2, February 2015 ISSN 1324-6012 The newsletter of The LEAD (Lead Education and Abatement Design) Group Inc. PO Box 161 Summer Hill NSW 2130 Australia Ph: (02) 9716 0014, Email www.lead.org.au/cu.html Web:www.lead.org.au/ www.leadsafeworld.com Editor-in-Chief: Elizabeth O’Brien, Editorial Team: Rocky Huang, Mish Calvert Combating the Silent Epidemic This issue is about providing you with information to combat lead poisoning. From nutritional information to finding out how to stay lead safe by paying attention to the different sources of lead contamination and ways that lead can enter the body of you and your family and taking the appropriate action to combat these threats. It is up to us, every single one of us, to combat lead in our local environment and together we can work towards a lead-safe future. To do this, The LEAD Group aims to arm you with information as well as provide you with the tools to detect and take further action about the lead in your surroundings. If you haven't already, please visit our shop (http://www.leadsafeworld.com/shop/) and become a member / partner and join our cause for a lead-safe future. Additionally, we highly recommend that you check out our newest project 'The Blood Lead Challenge (http://www.leadsafeworld.com/wp-content/uploads/2014/10/Blood-Lead- Challenge.pdf)! You can find more information about the articles in this issue of LEAD Action News in the Editorial. VAP Entry: Our children heading for a lead-safe world. -

Annual Report of the Director of the Mint
- S. Luriºus vsº ANNUAL REPORT Of the Director of the N/int for the fiscal year ended June 30, 1970. ANNUAL REPORT of the Director of the Mint for the fiscal year ended June 30 1970 DEPARTMENT OF THE TREASURY DOCUMENT NO. 3253 Director of the Mint U.S. GOVERNMENT PRINTING OFFICE WASHINGTON : 1971 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price $1 (paper cover) Stock Number 4805–0009 LETTER OF TRANSMITTAL DEPARTMENT OF THE TREASURY, BUREAU OF THE MINT, Washington, D.C., April 29, 1971. SIR: I have the honor to submit the Ninety-eighth Annual Report of the Director of the Mint, since the Mint became a Bureau within the Department of the Treasury in 1873. Annual reports of Mint activities have been made to the Secretary of the Treasury since 1835, pursuant to the act of March 3, 1835 (4 Stat. 774). Annual reports of the Mint have been made since it was established in 1792. This report is submitted in compliance with Section 345 of the Revised Statutes of the United States, 2d Edition (1878), 31 U.S.C. 253. It includes a review of the operations of the mints, assay offices, and the bullion depositories for the fiscal year ended June 30, 1970. Also contained in this edition are reports for the calendar year 1969 on U.S. gold, silver, and coinage metal production and the world's monetary stocks of gold, silver, and coins. MARY BROOKs, Director of the Mint. Hon. JoHN B. Con NALLY, Secretary of the Treasury. -

Section 1 Introduction to Alloy Phase Diagrams
Copyright © 1992 ASM International® ASM Handbook, Volume 3: Alloy Phase Diagrams All rights reserved. Hugh Baker, editor, p 1.1-1.29 www.asminternational.org Section 1 Introduction to Alloy Phase Diagrams Hugh Baker, Editor ALLOY PHASE DIAGRAMS are useful to exhaust system). Phase diagrams also are con- terms "phase" and "phase field" is seldom made, metallurgists, materials engineers, and materials sulted when attacking service problems such as and all materials having the same phase name are scientists in four major areas: (1) development of pitting and intergranular corrosion, hydrogen referred to as the same phase. new alloys for specific applications, (2) fabrica- damage, and hot corrosion. Equilibrium. There are three types of equili- tion of these alloys into useful configurations, (3) In a majority of the more widely used commer- bria: stable, metastable, and unstable. These three design and control of heat treatment procedures cial alloys, the allowable composition range en- conditions are illustrated in a mechanical sense in for specific alloys that will produce the required compasses only a small portion of the relevant Fig. l. Stable equilibrium exists when the object mechanical, physical, and chemical properties, phase diagram. The nonequilibrium conditions is in its lowest energy condition; metastable equi- and (4) solving problems that arise with specific that are usually encountered inpractice, however, librium exists when additional energy must be alloys in their performance in commercial appli- necessitate the knowledge of a much greater por- introduced before the object can reach true stabil- cations, thus improving product predictability. In tion of the diagram. Therefore, a thorough under- ity; unstable equilibrium exists when no addi- all these areas, the use of phase diagrams allows standing of alloy phase diagrams in general and tional energy is needed before reaching meta- research, development, and production to be done their practical use will prove to be of great help stability or stability. -

Multi-Response Optimization of Process Parameters by Taguchi Grey Relational Analysis for Dissimilar Thickness Friction Stir Process Corner Weld Aa5086 Alloy
Journal of Engineering Science and Technology Vol. 13, No. 10 (2018) 3297 - 3312 © School of Engineering, Taylor’s University MULTI-RESPONSE OPTIMIZATION OF PROCESS PARAMETERS BY TAGUCHI GREY RELATIONAL ANALYSIS FOR DISSIMILAR THICKNESS FRICTION STIR PROCESS CORNER WELD AA5086 ALLOY MANIGANDAN KRISHNAN*, SENTHILKUMAR SUBRAMANIAM School of Mechanical Engineering, VIT University, Vellore, India *Corresponding Author: [email protected] Abstract This paper presents the multi-response optimization of friction stir corner welding process for dissimilar thickness AA5086 aluminium alloy plates. The corner joint of AA5086 aluminium alloy plates of thicknesses of 6 mm and 4 mm was welded by Friction Stir Welding (FSW) process. The FSW experiments were conducted agreeing to the L9 orthogonal array. Three FSW process parameters: tool traverse speed (100, 150 and 190 mm/min), rotational speed (900, 1000 and 1100 rev/min), and plunge depth (0.1, 0.2, and 0.3 mm) were related with weld tensile strength and hardness. The Analysis of Variance (ANOVA) was used to determine the percentage contribution of each input parameter on the weld quality. Taguchi Grey Relational Analysis is used to optimize and order the FSW process parameters. Conferring to the results of the analyses, the optimal welding condition was determined as 1000 rev/min for tool rotational speed, 150 mm/min for traverse speed and 0.1 mm for tool plunge depth. The percentage contribution of the traverse speed (54%) revealed a significant influence compared to tool rotational speed (21%) and plunge depth (13%). The microstructures of various zones were observed and analysed. Tensile tests were conducted and the fracture was observed at heat affected zones for all the joints.