Aluminum and Its Alloys
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Connor Final Highlighted
Preliminary Development of a PPAM Actuated Pediatric Prosthetic Ankle Connor McNamara-Spackman A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science (Bioengineering) at the University of Otago, Dunedin, New Zealand. February 2021 Abstract The purpose of this research was to develop a preliminary design of a powered pediatric prosthetic ankle. Previous research identified the health risk of improper gait cycle and the lack of powered prosthetic ankle options for children. Costs for powered prosthetic ankles are too high (upwards of $5000 NZD), the sizes are too large and the weight is too significant for a child to benefit from. Current technologies for ankle joint actuation and materials for the prosthetic structure were evaluated and a conclusion of utilizing PPAMs was chosen due to their ability to generate the required 300 N of contraction force. CAD was used to model the structure of a prosthetic ankle and evaluate the FOS of the different material combinations while under static loading and fatigue simulations. HDPE and UHMWPE failed to withstand the simulations, while the aluminium alloy and stainless steel showed minimal faults from the simulations. MatLab was used to simulate the desired PPAM dimensions of 100 mm to determine the contraction force and contraction percentage that can be generated by the PPAM. The smallest PPAM found in research was 110 mm and showed promising results from their mathematical modeling. The overall height of the prosthetic was no greater than 110 mm and the membrane length of the PPAM was no greater than 100 mm, while successfully producing more than 300 N during contraction. -

Reduction of Out-Of-Plane Distortion in Fillet Welded High Strength Aluminum
Calhoun: The NPS Institutional Archive Theses and Dissertations Thesis Collection 1974 Reduction of out-of-plane distortion in fillet welded high strength aluminum. Henry, Robert W. Massachusetts Institute of Technology http://hdl.handle.net/10945/17204 REDUCTION OF OUT-OF-PLANE DISTORTION IN FILLET WELDED HIGH STRENGTH ALUMINUM Robert W. Henry il Postgraduate School vionterey, California 93940 REDUCTION OF OUT-OF-PLANE DISTORTION IN FILLET WELDED HIGH STRENGTH ALUMINUM BY Robert W. Henry B.S., U.S. Coast Guard Academy (1969) SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREES OF MASTER OF SCIENCE IN OCEAN ENGINEERING AND MASTERS OF SCIENCE IN MECHANICAL ENGINEERING at the Massachusetts Institute of Technology May, 1974 Tii-c DUDLEY KNOX LIBRARY ^STGRADL'ATE SCHOOU - 93940 REDUCTION OF OUT-OF-PLANE DISTORTION IN FILLET WELDED HIGH STRENGTH ALUMINUM by Robert W. Henry Submitted to the Department of Ocean Engineering in May, 1974, in partial fulfillment of the requirements for the degree of Master of Science in Ocean Engineering and Masters of Science in Mechanical Engineering. ABSTRACT Out-of-plane distortion caused by angular changes at fillet welds in aluminum structural panels was examined from two viewpoints. In the first phase of this work a series of experiments was conducted to examine elastic-plastic prestraining of test specimens to be fillet welded as a means of reducing out-of -plane distortion. Data gathered from these tests was correlated with previous experiments in the use of aluminum. A guide in the use of elastic-plastic prestrain- ing for 3/8" and 1/2" was developed. In phase two of this work a two-dimensional program was adapted to the structural aluminum panels used in phase one and tested for accuracy. -

Alcotec Aluminum Technical Guide
AlcoTec Aluminum Technical Guide Contents AlcoTec Aluminum Wire & Equipment Technical Guide Table of Contents AlcoTec Aluminum Wire & Equipment Technical Guide ......................................................................................................... 1 Table of Contents ................................................................................................................................................................ 1 Environmental Health and Safety ......................................................................................................................................... 3 Technical Services Heat Treatable & Non-Heat Treatable Base & Fillers ............................................................................................................. 6 Filler Alloys: Chemical Composition Limits & Physical Properties ......................................................................................... 7 Conversion Factors ............................................................................................................................................................ 7 Welded Joint Strength ......................................................................................................................................................... 8 Typical Tensile Properties - Groove Welds ............................................................................................................................ 9 Weld Profiles ..................................................................................................................................................................... -

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -
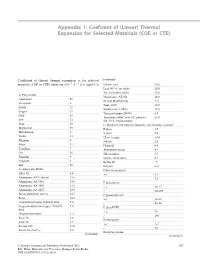
Linear) Thermal Expansion for Selected Materials (COE Or CTE
Appendix 1: Coefcient of (Linear) Thermal Expansion for Selected Materials (COE or CTE) Coefficient of (linear) thermal expansion, α, for selected (continued) − − materials (COE or CTE) (units are ×10 6 °C 1 (i.e. ppm/°C)) Indium–lead 33.0 Lead (95 %) tin solder 28.0 Tin–lead solder 60/40 25.0 A. Pure metals Magnesium, AZ31B 26.0 Aluminium 25 Ni-clad Molybdenum 5–6 Chromium 6 Steel, 1020 12.0 Cobalt 12 Stainless steel (18-8) 17.0 Copper 17 Tungsten/copper (90/10) 6.5 Gold 14 Aluminium MMC with SiC particles 6–14 Iron 12 (80–50 % reinforcement) Lead 29 C. Insulators and substrate materials (for electronic systems)a Magnesium 25 E glass 5.5 Molybdenum 5 S glass 2.6 Nickel 13 Glass–ceramic >3.0 Platinum 9 Silicon 2.6 Silver 19 Diamond 0.9 Tantalum 7 Aluminium nitride 4.5 Tin 20 Silicon nitride 3.7 Titanium 9 Quartz, fused silica 0.5 Tungsten 5 Kevlar 49 –5 Zinc 35 Beryllia 6–9 B. Alloys and MMCs Cubic boron nitride Alloy 42 4.4 x–y 3.7 Aluminium (40 % silicon) 13.5 z 7.2 Aluminium, AA 6061 23.6 E glass/epoxy Aluminium, AA 3003 23.2 x–y 14–17 Aluminium, AA 2017 22.9 z 80–280 Boron aluminium (20 %) 12.7 E glass/polyimide Brass 18.0 x–y 12–16 Copper/invar/copper 20/60/20 thick 5.8 z 40–80 Copper/molybdenum/copper 20/60/20 7.0 E glass/PTFE thick x–y 24 Graphite/aluminium 4–6 z 260 Invar 36 1.6 Kevlar/epoxy Invar 42 4.5 x–y 5–7 Inconel 600 13.0 z 70 Kovar (Fe–Ni–Co) 5.0 (continued) Kevlar/polyimide (continued) © Springer International Publishing Switzerland 2016 557 B.D. -

Characteristics of Al-Si Alloys with High Melting Point Elements for High Pressure Die Casting
materials Article Characteristics of Al-Si Alloys with High Melting Point Elements for High Pressure Die Casting Tomasz Szymczak 1,* , Grzegorz Gumienny 1,* , Leszek Klimek 2 , Marcin Goły 3 , Jan Szymszal 4 and Tadeusz Pacyniak 1 1 Department of Materials Engineering and Production Systems, Lodz University of Technology, 90-924 Lodz, Poland; [email protected] 2 Institute of Materials Science and Engineering, Lodz University of Technology, 90-924 Lodz, Poland; [email protected] 3 Department of Physical & Powder Metallurgy, AGH University of Science and Technology, 30-059 Krakow, Poland; [email protected] 4 Department of Technical Sciences and Management, University of Occupational Safety Management in Katowice, 40-007 Katowice, Poland; [email protected] * Correspondence: [email protected] (T.S.); [email protected] (G.G.); Tel.: +48-426312276 (T.S.); +48-426312264 (G.G.) Received: 9 October 2020; Accepted: 29 October 2020; Published: 29 October 2020 Abstract: This paper is devoted to the possibility of increasing the mechanical properties (tensile strength, yield strength, elongation and hardness) of high pressure die casting (HPDC) hypoeutectic Al-Si alloys by high melting point elements: chromium, molybdenum, vanadium and tungsten. EN AC-46000 alloy was used as a base alloy. The paper presents the effect of Cr, Mo, V and W on the crystallization process and the microstructure of HPDC aluminum alloy as well as an alloy from the shell mold. Thermal and derivative analysis was used to study the crystallization process. The possibility of increasing the mechanical properties of HPDC hypoeutectic alloy by addition of high-melting point elements has been demonstrated. -

Tribological Behaviours Influenced by Surface Coatings and Morphology
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 7-11-2015 Tribological Behaviours Influenced yb Surface Coatings and Morphology Guang Wang University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Wang, Guang, "Tribological Behaviours Influenced yb Surface Coatings and Morphology" (2015). Electronic Theses and Dissertations. 5305. https://scholar.uwindsor.ca/etd/5305 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang A thesis Submitted to the Faculty of Graduate Studies through the Department of Mechanical, Automotive & Materials Engineering in Partial Fulfillment of the Requirements for the Degree of Master of Applied Science at the University of Windsor Windsor, Ontario, Canada 2015 ©2015 Guang Wang Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang APPROVED BY: Dr. -

Aluminum Sheet / Plate
Advanced Metal Technology Co.,Limited ALUMINUM PRODUCTS SERIES Advanced Metal Technology Co.,Limited 1 Hongnan Mansion, No.939 Jinqiao Road, Shanghai, China Post Code:200136 Tel: 0086-21-61623132 Fax: 0086-21-61622559 Henghua Technology Park,NAO.58dXiuvxi Raoand, WcuXei,Chdina Metal Technology Co.,Limited Post Code:214000 Tel: 0086-510-85192612 Fax: 0086-510-85192613 Flat/RM 1205, Tai Sang Bank Building, 130-132 Des Voeux Road Central, HK, China Advanced Metal Technology Co.,Limited CONTENT INTRODUCTION...............................................................................................................................3 ALUMINUM SHEET / PLATE..........................................................................................................3 1000 Series 1050 1060 1070 1100..............................................................................................6 5000 Series 5050 5054 5083 5454..............................................................................................7 6000 Series 6010 6011 6061 6062 6063.....................................................................................8 7000 Series 7005 7A04 7A09 7050 7075...................................................................................9 Aluminum Composite Sheet..................................................................................................... 10 Roof Ceiling.............................................................................................................................. 12 Air Plane Plate...........................................................................................................................14 -

Isothermes Kurzzeitermüdungsverhalten Der
i N Osama Khalil u2Mg1,5 C l A ISOTHERMES KURZZEITERMÜDUNGS- VERHALTEN DER HOCHWARMFESTEN ALUMINIUM-KNETLEGIERUNG 2618A verhalten von (AlCu2Mg1,5Ni) SCHRIFTENREIHE DES INSTITUTS FÜR ANGEWANDTE MATERIALIEN BAND 34 Isothermes Kurzzeitermüdungs O. KHALIL Osama Khalil Isothermes Kurzzeitermüdungsverhalten der hochwarm- festen Aluminium-Knetlegierung 2618A (AlCu2Mg1,5Ni) Schriftenreihe des Instituts für Angewandte Materialien Band 34 Karlsruher Institut für Technologie (KIT) Institut für Angewandte Materialien (IAM) Eine Übersicht über alle bisher in dieser Schriftenreihe erschienenen Bände finden Sie am Ende des Buches. Isothermes Kurzzeitermüdungs- verhalten der hochwarmfesten Aluminium-Knetlegierung 2618A (AlCu2Mg1,5Ni) von Osama Khalil Dissertation, Karlsruher Institut für Technologie (KIT) Fakultät für Maschinenbau Tag der mündlichen Prüfung: 15. Januar 2014 Impressum Karlsruher Institut für Technologie (KIT) KIT Scientific Publishing Straße am Forum 2 D-76131 Karlsruhe KIT Scientific Publishing is a registered trademark of Karlsruhe Institute of Technology. Reprint using the book cover is not allowed. www.ksp.kit.edu This document – excluding the cover – is licensed under the Creative Commons Attribution-Share Alike 3.0 DE License (CC BY-SA 3.0 DE): http://creativecommons.org/licenses/by-sa/3.0/de/ The cover page is licensed under the Creative Commons Attribution-No Derivatives 3.0 DE License (CC BY-ND 3.0 DE): http://creativecommons.org/licenses/by-nd/3.0/de/ Print on Demand 2014 ISSN 2192-9963 ISBN 978-3-7315-0208-1 DOI 10.5445/KSP/1000040485 Isothermes Kurzzeitermüdungsverhalten der hochwarmfesten Aluminium-Knetlegierung 2618A (AlCu2Mg1,5Ni) Zur Erlangung des akademischen Grades Doktor der Ingenieurwissenschaften an der Fakultät für Maschinenbau des Karlsruher Institut für Technologie (KIT) genehmigte Dissertation von Dipl.-Ing. -

Statistical Analysis of the Optical Interferometry of Pitting Process in Aluminum 3003 Sheets Exposed to Saline Environment
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Available online at www.sciencedirect.com ScienceDirect Procedia Materials Science 8 ( 2015 ) 82 – 90 International Congress of Science and Technology of Metallurgy and Materials, SAM - CONAMET 2013 Statistical Analysis of the Optical Interferometry of Pitting Process in Aluminum 3003 Sheets Exposed to Saline Environment Solange Y. Paredes-Dugarte, Benjamín Hidalgo-Prada Material Science Department, Materials Characterization Laboratory. Research Institute of Biomedicine and Applied Sciences “Dra. Susan Tai”, University Ave., Cumaná, Sucre 6101, Venezuela. Abstract In this study, a statistical evaluation was made of the susceptibility to pitting corrosion, using the pitting factor criteria. Specimens were cut in size of 15 cm x10 cm of AA3003 aluminium sheet of temper H14, H16 and H18 of national production. Afterwards, they were exposed to a salt spray during 72, 144, 216, 288 and 360 hours continually, according to ASTM B117 standard. After salt spray test was observed pitting attack in all specimens regardless of the exposure time and the degree of deformation (temper) of material. The surfaces of the corroded specimens were analyzed by optical interferometry. The parameters evaluated in each field were: roughness (Rms), peak-valley distance (PV) and the lowest point of all peaks (V). A pitting factor of the order of 4 was calculated, indicating a highly localized corrosion process for this commercial aluminium alloy 3003 in saline environment. © 20152014 TheThe Authors. Authors. Published Published by Elsevierby Elsevier Ltd. LtdThis. is an open access article under the CC BY-NC-ND license Selection(http://creativecommons.org/licenses/by-nc-nd/4.0/ and peer-review under responsibility). -

Abstracts from the Scientific and Technical Press Titles And
December, ig4j Abstracts from the Scientific and Technical Press " (No. 117. October, 1943) AND Titles and References of Articles and Papers Selected from Publications (Reviewed by R.T.P.3) TOGETHER WITH List of Selected Translations (No. 63)' London : "THE ROYAL AERONAUTICAL SOCIETY" with which is incorporated "The Institution of Aeronautical Engineers" 4, Hamilton Place, W.I Telephone: Grosvenor 3515 (3 lines) ABSTRACTS FROM THE SCIENTIFIC AND TECHNICAL PRESS. Issued by the Directorate's of Scientific Research and Technical Development, Ministry of Air craft Production. (Prepared by R.T.P.3.) No. 117. OCTOBER, 1943. Notices and abstracts from the Scientific and Technical Press are prepared primarily for_ the information of Scientific and Technical Staffs. Particular attention is paid to the work carried out in foreign countries, on the assumption that the more accessible British work (for example that published by the Aeronautical Research Committee^ is already known to these Staffs. Requests from scientific and technical staffs for further information of transla tions should be addressed to R.T,P.3, Ministry of Aircraft Production, and not to the Royal Aeronautical Society. Only a limited number of the articles quoted from foreign journals are trans lated and usually only the original can be supplied on loan. If, however, translation is required, application should be made in writing to R.T.P.3, the requests being considered in accordance with existing facilities. ' NOTE.—As far as possible, the country of origin quoted in the items refers to the original source. The Effect of Nitrogen on the Properties of Certain Austenitic Valve Steels. -

Metallurgical Abstracts (General and Non-Ferrous)
METALLURGICAL ABSTRACTS (GENERAL AND NON-FERROUS) Volume 2 1935 Part 13 I —PROPERTIES OF METALS (Continued from pp. 553-568.) Refined Aluminium. Robert GaDeau (Metallurgist (Suppt. to Engineer), 1936, 11, 94-96).—Summary of a paper presenteD to the Congrès Inter nationale Des Mines, De la Métallurgie, et De la Géologie Appliquée, Paris. See Met. Abs., this vol., pp. 365 anD 497.—R. G. _ On the Softening and Recrystallization of Pure Aluminium. ------ (A lu minium, 1935, 17, 575-576).—A review of recent work of Calvet anD his collaborators ; see Met. Abs., this vol., pp. 453, 454. A. R. P. *Some Optical Observations on the Protective Films on Aluminium in Nitric, Chromic, and Sulphuric Acids. L. TronstaD anD T. HbverstaD (Trans. Faraday Soc., 1934, 30, 362-366).—The optical properties of natural films on aluminium were measureD in various solutions anD their change with time of immersion observeD. Little change occurs in such films in chromic aciD solutions with or without chloriDe ; the films are not protective in concentrateD sulphuric aciD, anD in concentrateD nitric aciD the protective films are alternately DissolveD anD re-formeD. The mean thickness of natural films on aluminium is 100 p. or more than 10 times as thick as those on iron.—A. R. P. *Light from [Burning] Aluminium and Aluminium-Magnésium [Alloy], J. A. M. van Liempt anD J. A. De VrienD (Bee. trav. chim., 1935, 54, 239-244). „ . —S. G. ’"Investigations Relating to Electrophotophoresis Exhibited by Antimony Gisela Isser anD AlfreD Lustig (Z . Physik, 1935, 94, 760-769).—UnchargeD submicroscopic particles subjecteD to an electric fielD in an intense beam of light are founD to move either in the Direction of, or against, the fielD.