Branched Chain Saturated Alcohols When Used As Fragrance Ingredients Q
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enlarging Knowledge on Lager Beer Volatile Metabolites Using Multidimensional Gas Chromatography
foods Article Enlarging Knowledge on Lager Beer Volatile Metabolites Using Multidimensional Gas Chromatography Cátia Martins 1 , Tiago Brandão 2, Adelaide Almeida 3 and Sílvia M. Rocha 1,* 1 Departamento de Química & LAQV-REQUIMTE, Universidade de Aveiro, Campus Universitário Santiago, 3810-193 Aveiro, Portugal; [email protected] 2 Super Bock Group, Rua do Mosteiro, 4465-703 Leça do Balio, Portugal; [email protected] 3 Departamento de Biologia & CESAM, Universidade de Aveiro, Campus Universitário Santiago, 3810-193 Aveiro, Portugal; [email protected] * Correspondence: [email protected]; Tel.: +351-234-401-524 Received: 30 July 2020; Accepted: 6 September 2020; Published: 11 September 2020 Abstract: Foodomics, emergent field of metabolomics, has been applied to study food system processes, and it may be useful to understand sensorial food properties, among others, through foods metabolites profiling. Thus, as beer volatile components represent the major contributors for beer overall and peculiar aroma properties, this work intends to perform an in-depth profiling of lager beer volatile metabolites and to generate new data that may contribute for molecules’ identification, by using multidimensional gas chromatography. A set of lager beers were used as case-study, and 329 volatile metabolites were determined, distributed over 8 chemical families: acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols. From these, 96 compounds are reported for the first time in the lager beer volatile composition. Around half of them were common to all beers under study. Clustering analysis allowed a beer typing according to production system: macro- and microbrewer beers. Monoterpenic and sesquiterpenic compounds were the chemical families that showed wide range of chemical structures, which may contribute for the samples’ peculiar aroma characteristics. -

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -
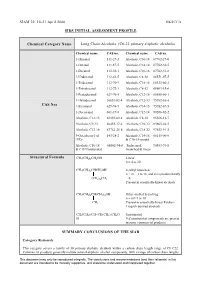
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Volatile Organic Compounds from Books
1 Measuring the emission of volatile organic compounds from books Velson Horie Research Project Manager The British Library What is happening to our books? presevation of folding endurance DP X. Zou, T. Uesaka, N. Gurnagul, Prediction of paper permanence by accelerated aging. Part I: Kinetic analysis of the 3 aging process , Cellulose, 1996, 3, 243-267. A Few Statistics •Formal beginning in 1753 as the library of The British Museum •The British Library formed in 1973 from many collections •New St Pancras building opened in 1998 •150m collection items on 640km of shelves, •£131m budget, 1900 staff 4 Additional Storage Programme - Boston Spa •7 million collection items •263 km, 12,000 tonne of stock •Reduced oxygen (16%) •Robotic book handling •What are the long term effects? 5 Preserving Newspapers •33 km of stock •5,300 tonne of stock •1.4 tonne/y VOC production •3,800 years till all evaporated 6 Major UK libraries and archives Cambridge University Library (CUL) 7m printed items The British Library (BL) 150m items National Library of Scotland (NLS) 14m items National Library of Wales (NLW) 6m printed items Oxford University Library(OULS) 11m items Trinity College Dublin Library (TCD) 4m printed items The National Archives (TNA) National Archives of Scotland (NAS) 7 Condition assessment Preservation Assessment Survey Strength Colour pH Molecular weight Furnish SurveNIR VOCs 8 The “real thing” is important to people E-books sales have been slow to take off. CafeScribe is sending every e-textbook purchaser a scratch and sniff sticker with a musty “old book” smell. By placing these stickers on their computers, they can give their e-books the same musty book smell they know and love from used textbooks. -

Reactions of Alcohols & Ethers 1
REACTIONS OF ALCOHOLS & ETHERS 1. Combustion (Extreme Oxidation) alcohol + oxygen carbon dioxide + water 2 CH3CH2OH + 6 O2 4 CO2 + 6 H2O 2. Elimination (Dehydration) ° alcohol H 2 S O 4/ 1 0 0 C alkene + water H2SO4/100 ° C CH3CH2CH2OH CH3CH=CH2 + H2O 3. Condensation ° excess alcohol H 2 S O 4 / 1 4 0 C ether + water H2SO4/140 ° C 2 CH3CH2OH CH3CH2OCH2CH3 + H2O 4. Substitution Lucas Reagent alcohol + hydrogen halide Z n C l2 alkyl halide + water ZnCl2 CH3CH2OH + HCl CH3CH2Cl + H2O • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcohol→ turns cloudy immediately (the alkyl halide is not soluble in water and precipitates out) • Secondary Alcohol → turns cloudy after 5 minutes • Primary Alcohol → takes much longer than 5 minutes to turn cloudy 5. Oxidation • Uses an oxidizing agent such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7). • This reaction can also be used as a qualitative test for the different types of alcohols because there is a distinct colour change. dichromate → chromium 3+ (orange) → (green) permanganate → manganese (IV) oxide (purple) → (brown) Tertiary Alcohol not oxidized under normal conditions CH3 KMnO4 H3C C OH NO REACTION K2Cr2O7 CH3 tertbutyl alcohol Secondary Alcohol ketone + hydrogen ions H O KMnO4 H3C C CH3 + K2Cr2O7 C + 2 H H3C CH3 OH propanone 2-propanol Primary Alcohol aldehyde + water carboxylic acid + hydrogen ions O KMnO4 O H H KMnO H H 4 + CH3CH2CH2OH C C C H C C C H + 2 H K2Cr2O7 + H2O K Cr O H H H 2 2 7 HO H H 1-propanol propanal propanoic acid 6. -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

Soln-2212-PT3-S20-Ch-16-18.Pdf
1. 2. H2SO4 H2SO4 H2SO41) BH3, THF 1) BH3, THF 1) BH3, THF - - - 2) OH , H2O2, H2O2) OH , H2O2, H2O 2) OH , H2O2, H2O OH OH OH 3. OH OH OH H2SO4 1) BH3, THF - 2) OH , H2O2, H2O OH PBr3 PBr3 PBr3 Pyridine Pyridine Pyridine OH PBr3 Pyridine O O O Mg Mg Mg HO HO HO1) 1) 1) Ether Ether Ether 2) HCl 2) HCl 2) HCl Br Br Br MgBr MgBr MgBr O Mg HO 1) Ether 2) HCl Br 4. MgBr **This reaction reduces an aldehyde to a primary alcohol and a ketone to a secondary alcohol. Since only one EQ of reagent is given the most reactive functional group will be reduced (in this case the aldehyde) **ketones are less reactive than aldehydes and the more steric hindrance surrounding the ketone the less reactive it is. Note: NaBH4 reduces ketones but is too weak to reduce an ester/double/triple bonds 5. ** With these reagents a tertiary amide will be reduced to a tertiary amine, a secondary amide will be reduced to a secondary amine, and a primary amide will be reduced to a primary amine 6. ** Recall from chapter 7, OChem 1 H2 and Lindlars catalyst always reduces an alkyne to a CIS ALKENE ** Li/Na and liquid NH3 always reduces an alkyne to a TRANS ALKENE 7. **A ketone/aldehyde reacts with primary amines in trace acid to produce an imine, and reacts with a secondary amine in trace acid to produce an enamine (double bond forms between the alpha and beta carbons) **Sodium cyanoborohydride (NaBH3CN) is used to reduce imines/enamines back to their respective secondary/tertiary amines 8. -

Chapter 19 the Chemistry of Aldehydes and Ketones. Addition Reactions
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 19 The Chemistry of Aldehydes and Ketones. Addition Reactions Solutions to In-Text Problems 19.1 (b) (d) (e) (g) 19.2 (a) 2-Propanone (d) (E)-3-Ethoxy-2-propenal (f) 4,4-Dimethyl-2,5-cyclohexadienone 19.3 (b) 2-Cyclohexenone has a lower carbonyl stretching frequency because its two double bonds are conjugated. 19.4 (b) The compound is 2-butanone: (c) The high frequency of the carbonyl absorption suggests a strained ring. (See Eq. 19.4, text p. 897.) In fact, cyclobutanone matches the IR stretching frequency perfectly and the NMR fits as well: 19.6 The structure and CMR assignments of 2-ethylbutanal are shown below. The two methyl groups are chemically equivalent, and the two methylene groups are chemically equivalent; all carbons with different CMR chemical shifts are chemically nonequivalent. INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 19 2 19.7 (a) The double bonds in 2-cyclohexenone are conjugated, but the double bonds in 3-cyclohexenone are not. Consequently, 2-cyclohexenone has the UV spectrum with the greater lmax. 19.9 Compound A, vanillin, should have a p T p* absorption at a greater lmax when dissolved in NaOH solution because the resulting phenolate can delocalize into the carboxaldehyde group; the resulting phenolate from compound B, isovanillin, on the other hand, can only delocalize in the aromatic ring. 19.11 The mass spectrum of 2-heptanone should have major peaks at m/z = 43 (from a-cleavage), 71 (from inductive cleavage), and 58 (from McLafferty rearrangement). -

(12) Patent Application Publication (10) Pub. No.: US 2011/0028732 A1 Trauth Et Al
US 2011 0028732A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0028732 A1 Trauth et al. (43) Pub. Date: Feb. 3, 2011 (54) NITRATED HYDROCARBONS, (86). PCT No.: PCT/US2009/0399.01 DERIVATIVES, AND PROCESSES FOR THEIR MANUFACTURE S371 (c)(1), (2), (4) Date: Sep. 27, 2010 (75) Inventors: Daniel M. Trauth, Crystal Lake, IL Related U.S. Application Data (US); George D. Green, Cary, IL (US); Raymond J. Swedo, Mt. (60) Eyal application No. 61/045.380, filed on Apr. Prospect, IL (US); Richard L. s James, Eros, LA (US); Ian A. Publication Classification Tomlinson, Midland, MI (US) (51) Int. Cl. C07D 263/04 (2006.01) Correspondence Address: C07C 205/05 (2006.01) The Dow Chemical Company C07C 205/01 (2006.01) P.O. BOX 1967, 2040 Dow Center CD7C 205/06 (2006.01) Midland, MI 48641 (US) C07C 215/02 (2006.01) C07C 239/08 (2006.01) (73) Assignees: ANGUS CHEMICAL (52) U.S. Cl. ......... 548/215; 7.3,So COMPANY, Buffalo Grove, IL s s (US): GLOBAL (57) ABSTRACT TECHNOLOGIES INC. , MidlandM1Clland, Provided is a process for the formation of nitrated compounds MI (US) by the nitration of hydrocarbon compounds with- dilute- - nitric acid. Also provided are processes for preparing industrially (21) Appl. No.: 12/934,817 useful downstream derivatives of the nitrated compounds, as well as novel nitrated compounds and derivatives, and meth (22) PCT Filed: Apr. 8, 2009 ods of using the derivatives in various applications. US 2011/0028732 A1 Feb. 3, 2011 NITRATED HYDROCARBONS, 0009. The invention further provides methods of using the DERIVATIVES, AND PROCESSES FOR THEIR nitrated hydrocarbons and derivatives thereof in various MANUFACTURE applications. -

Cambridge CB2 3EG (Received 14 June 1990)
Journal of Physiology (1991), 437, pp. 431-448 431 With 11 figures Printed in Great Britain ACTIONS OF n-ALCOHOLS ON NICOTINIC ACETYLCHOLINE RECEPTOR CHANNELS IN CULTURED RAT MYOTUBES BY R. D. MURRELL*, M. S. BRAUNt AND THE LATE D. A. HAYDON From the Physiological Laboratory, University of Cambridge, Downing Street, Cambridge CB2 3EG (Received 14 June 1990) SUMMARY 1. The actions of the n-alcohols from pentanol to dodecanol on nicotinic acetylcholine receptor (nAChR) channels were investigated by recording single ACh- activated channel activity from inside-out membrane patches isolated from cultured rat myotubes. Alcohols were applied to the cytoplasmic side of the membrane; aqueous concentrations ranged from 11 7 mM-pentanol to 0-02 mm-dodecanol. 2. The intermediate-chain alcohols (pentanol to octanol) caused channel currents to fluctuate between the fully open and closed state level so that openings occurred in bursts interrupted by brief gaps. Closed time distributions were fitted well with two exponential components, the fast component representing the closures within a burst. The number of gaps within a burst was dependent on alcohol concentration whereas gap duration was independent of concentration but increased with increasing chain length of the alcohol up to octanol. 3. Nonanol and decanol reduced the mean duration of bursts of openings but did not cause an increase in the number of short closed intervals within a burst. Beyond decanol there was a decline in the ability of the n-alcohols to affect channel function. A saturated solution of undecanol (0-07 mM) reduced the mean open time by 33+17 %, whereas a saturated solution of dodecanol had no significant effect. -

KUNDU-THESIS.Pdf
Acknowledgement I would like to thank my advisor Prof. Zachary T. Ball for teaching me everything I know in synthetic skills during my first year and guiding me through the rest of my time here at Rice. He has helped me better myself through constant constructive criticism, both in research and writing. I would also like to thank past and present Ball group members, Dr. Brian Popp, Dr. Alex Zaykov, Dr. Jessica Herron, Vincenzo Russo, Ramya Sambasivan, Cara Bovet, Zhen Chen, Dr. Jane Coughlin, Farrukh Vohidov, Matt Minus, Rob Ferguson, Julian Cooper. They have been a very good support in discussing research, exchanging ideas, and have become great pals in the last few years. Especially, Brian and Ramya’s encouragement in tough times is really valued. I thank my friends Rajkishore Barik and Meenu Adhikari for making Houston a second home. Again, Ramya Sambasivan for being a very good friend and always a willing ear to vent out frustrations associated with the graduate life and Avani Verma for bringing all kinds of fun in life and being so encouraging during my panic moments in thesis writing process. I would like to take this opportunity to express my gratitude and respect for my father Kuru Ram, and mother Amita Kundu, for the confidence and faith they always had in me, my sister Aruna Hajra who always supported me in my endeavors. I would like to thank my fiancée Soumya Sarkar, for his never wavering trust, love and support throughout my Ph.D in our 6 yr long, long- distance relation. Last but not least thanks to everyone who cared! -Rituparna Kundu Abstract Developing Dirhodium-Complexes for Protein Inhibition and Modification & Copper-Catalyzed Remote Chlorination of Alkyl-Hydroperoxides by Rituparna Kundu The work describes the development of a new class of protein-inhibitors for protein-protein interactions, based on metallopeptides comprised of a dirhodium metal center.