Adipose Stem Cells from Buccal Fat Pad and Abdominal Adipose Tissue for Bone Tissue Engineering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Buccal Fat Pad: Importance and Function
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 15, Issue 6 Ver. XIII (June. 2016), PP 79-81 www.iosrjournals.org The Buccal Fat Pad: Importance And Function Ulduz Zamani Ahari1, Hosein Eslami2, Parisa Falsafi*2, Aila Bahramian2, Saleh Maleki1 1. Post Graduate Student Of Oral Medicine, Dental Faculty, Tabriz University Of Medical Science, Tabriz, Iran. 2.Department Of Oral Medicine, Dental Faculty, Tabriz University Of Medical Science, Tabriz, Iran. ●Corresponding Author Email:[email protected] Abstract: The buccal fat is an important anatomic structure intimately related to the masticatory muscles, facial nerve , and parotid duct.while anatomically complex , an understanding of its boundaries will allow the surgeon to safely manipulate this structure in reconstructive and aesthetic procedures . Technically, the buccal fat pad is easily accessed and moblized . It is does not cause any noticeable defect in the cheek or mouth, and it can be performed in a very short time and without causing complication for the patient. The pedicled BFP graft is more resistant to infection than other kinds of free graft and will result in very success. The buccal fat pad has a satisfactory vascular supply , This property of graft and other properties will allow for the closure of defects that cannot be repaired by conventional procedures. The buccal fat pad exposed through an intraoral and buccal incision , has several clinical application including aesthetic improvement , close of oroantral and Oroantral defects reconstruction of surgical defects and palatal cleft defects and palatal cleft defects , reconstruction of maxilla and palatal defects. -
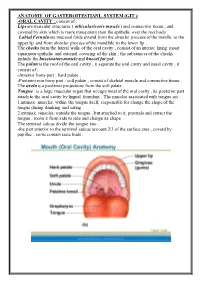
ANATOMY of GASTEROITESTIANL SYSTEM (GIT ): Consist of : ORAL CAVITY
ANATOMY OF GASTEROITESTIANL SYSTEM (GIT ): -ORAL CAVITY : consist of : Lips are muscular structures ( orbicularis oris muscle ) and connective tissue , and covered by skin which is more transparent than the epithelia over the rest body . Labial Fernula are mucosal folds extend from the alveolar process of the maxilla to the upper lip and from alveolar process of the mandible to the lower lip . The cheeks form the lateral walls of the oral cavity , consist of an interior lining moist squamous epithelia and external covering of the skin , the substances of the cheeks include the buccinators muscle and buccal fat pad. The palate is the roof of the oral cavity , it separate the oral cavity and nasal cavity , it consist of : -Anterior bony part : hard palate . -Posterior non bony part : soft palate , consist of skeletal muscle and connective tissue . The uvela is a posterior projections from the soft palate . Tongue : is a large muscular organ that occupy most of the oral cavity , its posterior part attach to the oral cavity by lingual frenulum . The muscles associated with tongue are : 1.intrinsic muscles: within the tongue itself, responsible for change the shape of the tongue during drinking and eating . 2.extrinsic muscles: outside the tongue , but attached to it, protrude and retract the tongue , move it from side to side and change its shape . The terminal sulcus divide the tongue into : -the part anterior to the terminal sulcus account 2/3 of the surface area , coverd by papillae , some contain taste buds . -the posterior one third is devoid of papillae , has few scattered taste buds , it has few small glands and lymphatic tissue (lingual tissue ) Teeth : adults have 32 teeth distributed in two dental arches, the maxillary arch , and the mandibular arch . -

Bichectomy: Achieving Aesthetic, Funcional and Psychological Results with a Simple Intraoral Surgical Procedure
Volume 1- Issue 2: 2017 DOI: 10.26717/BJSTR.2017.01.000205 Simone De Luccas. Biomed J Sci & Tech Res ISSN: 2574-1241 Opinion Open Access Bichectomy: Achieving Aesthetic, Funcional and Psychological Results with A Simple Intraoral Surgical Procedure Simone De Luccas* Dentist Surgeon, Brazil Received: July 13, 2017; Published: July 19, 2017 *Corresponding author: Simone De Luccas, Dentist Surgeon Rua Antonio Meneghetti, 226 - Sala 4 - Parque Terra Nova II, São Bernardo do Campo - SP, 09820-700, Brazil, Tel: ; Email: Opinion or female, with a round face. The best example is Nefertiti, the Bichectomy, or Bichat fat pad removal, is a surgical procedure Egyptian queen, that is still considered a beauty symbol with her indicated when there is excess volume on the middle third of the face (bellow the cheekbones / zygoma) giving it a chubby or round look. The ideal candidates are men and women who wish to achieve definedAlso and in prominentmy opinion, jaw the line. removal of Bichat fat pads do not a slimmer face appearance in this area, and it is also recommended contribute to ageing or future skin slack around the area. These are for patients with chronic cheek biting. Extra-oral photographs are the result of the normal ageing process, due to the loss of collagen part of the preoperative clinical assessment and will be compared and elastin and the shifting of fat tissues under the skin that are with photographs taken postoperative (immediately after the inevitable with age. This procedure changes the contour of the face with a decrease in bulk, resulting in less weight for the tissues to months), when the facial contour and volume reduction will be support, and, therefore, less sagging. -

Utilization of Pedicled Buccal Fat Pads for Coverage of the Lateral Relaxing Wound: a Review of Literature and a Case Series of 15 Patients
J Clin Exp Dent. 2018;10(5):e502-6. Erosive capacity of beverages on primary enamel Journal section: Oral Surgery doi:10.4317/jced.54797 Publication Types: Case Report http://dx.doi.org/10.4317/jced.54797 Utilization of pedicled buccal fat pads for coverage of the lateral relaxing wound: A review of literature and a case series of 15 patients Muhammad Ruslin, Andi S. Hajrah-Yusuf, Andi Tajrin, Lun-Jou Lo, Tymour Forouzanfar 1 Department of Oral and Maxillofacial Surgery/Oral Pathology, VU University Medical Center, P.O. Box 7057, 1007 MB, Am- sterdam, The Netherlands Correspondence: Department of Oral and Maxillofacial Surgery/Oral Pathology VU University Medical Center P.O. Box 7057, 1007 MB, Amsterdam, The Netherlands De Boelelaan 1117 1081 HV Amsterdam Ruslin M, Hajrah-Yusuf AS, Tajrin A, Lo LJ, Forouzanfar T. Utilization [email protected] of pedicled buccal fat pads for coverage of the lateral relaxing wound: A review of literature and a case series of 15 patients. J Clin Exp Dent. Received: 07/03/2018 2018;10(5):e502-6. Accepted: 11/04/2018 http://www.medicinaoral.com/odo/volumenes/v10i5/jcedv10i5p502.pdf Article Number: 54797 http://www.medicinaoral.com/odo/indice.htm © Medicina Oral S. L. C.I.F. B 96689336 - eISSN: 1989-5488 eMail: [email protected] Indexed in: Pubmed Pubmed Central® (PMC) Scopus DOI® System Abstract Background: The buccal fat pad (BFP) is an encapsulated mass originated from a specific fat tissue in various volume throughout the life of each person and BFP has been used in various surgeries as a source of useful graft material due to its easy accessibility and rich vascularization. -

Buccal Fat Pad As an Intra Oral Reconstruction Method in a Case of Oral Verrucous Carcinoma Dr
Case Report Buccal fat pad as an intra oral reconstruction method in a case of oral verrucous carcinoma Dr. Mohammad Asifur Rahman1* , Dr. Md. Harun - Ur – Rashid2, Dr. Ismat Ara Haider3 ABSTRACT AFFILIATION 1. Dr.Mohammad Asifur Rahman, BDS, MS(OMS), The buccal fat pad is a special type of fat tissue, which is Assistant professor, Department of Oral & Maxillofacial Surgery, Dhaka located anterior to the masseter muscle and deep to the Dental College Hospital, Dhaka. buccinator muscle. It provides gliding motion between 2. Dr. Md. Harun - Ur - Rashid, BDS, MS (OMS), muscles, protects the neurovascular bundles from injuries and Associate professor, Department of Oral & Maxillofacial Surgery, maintains facial convexity. The use of the buccal fat pad is Dhaka Dental College Hospital, Dhaka. promoted for the reconstruction of postsurgical intraoral 3. Dr. Ismat Ara Haider, BDS, DDS, MS (OMS), defects in verrucous carcinoma because of its easy to harvest Professor & Head, Department of Oral & Maxillofacial Surgery, and rich in vascular supply. Here we present a case of 65 years old male with verrucous Dhaka Dental College Hospital, Dhaka. carcinoma of left buccal mucosa and retro molar trigone came Article info. to the Department of Oral & Maxillofacial Surgery, Dhaka Dental College & Hospital, Dhaka, Bangladesh which was th Received: 20 August, 2018 successfully managed by surgical excision of lesion and Accepted: 23rd September, 2018 reconstruction with buccal fat pad. Volume: 8, Issue-2 October, 2018 DOI: https://doi.org/10.3329/updcj.v8i2.40383 KEY WORDS - Buccal fat pad, Verrucous carcinoma, Intra oral reconstruction INTRODUCTION © Authors retain copyright and grant the journal right of first Reconstruction of intraoral postsurgical defect is always publication with the work simultaneously licensed under Creative challenging because of its anatomical complexity and special Commons Attribution License CC - BY 4.0 that allows others to share type of intraoral tissues. -

Maxillofacial Infections
Maxillofacial Infections Dr Hazem Al-Ahmad B.D.S, MSc(Lon), F.D.S.R.C.S(Eng) Maxillofacial Infections • Odontogenic infections are usually mild and treated by antibiotics • A vestibular or a fascial space abcess is determined by the muscle attachment level to the point of infection perforation Microbiology • concentration of the organism • its virulence • environmental factors • host defense Steps in the management of odontogenic infections: • Determine the severity of infection. • Evaluate host defenses. • Decide on the setting of care • Treat surgically. • Support medically • Choose and prescribe antibiotic therapy • Administer the antibiotic properly • Evaluate the patient frequently. Severity of infection: • Anatomic location • Rate of progression, • Airway compromise. Anatomy Infections spread along the "path of least resistance". Fascial Spaces Bound by the fascial layers investing muscles of the body contain various structures are potential spaces Fascial Spaces Primary involvement spaces: Maxilla: canine, buccal, infratemporal Mandible: Submandibular, Sublingual, Submental Secondary spaces: Masseteric, pterygomandibular, superficial and deep temporal, Lateral pharyngeal, Retropharyngeal, prevertebral Canine Space • Sup Origin of levator muscles • Inf Orbicularis oris • Ant Skin, subQ • Post Maxilla • Med Levator labii alaquae nasii • Lat Zygomaticus major Canine Space • Canine root has a sufficient length to erode through the alveolar bone superior to the muscles of facial expressions. • Clinically, facial swelling that obliterates the nasolabial fold. • Spontaneous drainage occurs just inferior to the medial canthal ligament. Canine Space • Contains angular artery and vein, infraorbital foramen. • These provide a path of communication to cavernous sinus via ophthalmic vein, leading to cavernous sinusitis and brain abscess. • This is 2o to the fact that facial veins contain no valves. -

Bichat's Buccal Fat Pad Removal
Journal of Dental Health Oral Disorders & Therapy Case Report Open Access Bichat’s buccal fat pad removal: cheek reduction surgery Abstract Volume 7 Issue 4 - 2017 In the past few years there have been a growing number of procedures for the removal Caio Vinicius G Roman Torres,1,2 Adhmar of Bichat’s buccal fat pad, also called cheek reduction surgery. Bichat’s buccal fat pad Sani Junior,3 Juliana Cordeiro,4 Sergio may be used as part of the therapeutic procedure in cases of oroantral communication, 3 2,3 peri-orbital defects, congenital cleft palate, and plastic surgery of facial recontouring. The Marigny Filho, Rui Manuel Freire Sampaio, 1 purpose of this case report was to demonstrate the procedure of Bichat’s buccal fat pad, due Leticia C Cidreira Boaro, Angelica Castro to the frequent habit of biting the oral mucosa by the patient. Buccal fat pad removal is a Pimentel1 minor procedure, and the surgical technique is considered simple and safe if performed by 1Professor of Department of Post Graduation, Division of trained and experienced professionals. The postoperative can be compared to an extraction Implantology, School of Dentistry, University of Santo Amaro- of third molar, and the use of analgesics, anti-inflammatory controls properly any painful UNISA, Brazil 2 symptoms. The buccal fat pad removal should be carried out following a precise indication, Professor of Department of Graduation, Division of Dentistry, always with the patient conscious of the risks and benefits that may be obtained. University Metropolitan Santos-UNIMES, -

Comparison of Efficacy of Buccal Fat Pad and Collagen Membrane In
IJPCDR Rajbir K Randhawa et al 10.5005/jp-journals-10052-0110 ORIGINAL RESEARCH Comparison of Efficacy of Buccal Fat Pad and Collagen Membrane in Surgical Management of Oral Submucous Fibrosis 1Rajbir K Randhawa, 2Gagandeep S Randhawa, 3Saba Tiwari, 4KC Gupta, 5Anisha Maria, 6Mrinal Satpathy ABSTRACT Keywords: Buccal fat pad, Collagen membrane, Mouth opening, Oral submucous fibrosis. Background and objectives: Oral submucous fibrosis (OSMF) is a chronic progressive premalignant condition, characterized How to cite this article: Randhawa RK, Randhawa GS, Tiwari S, by gradual trismus of mouth. The study was done to compare Gupta KC, Maria A, Satpathy M. Comparison of Efficacy of the efficacy of buccal fat pad (BFP) and collagen membrane as Buccal Fat Pad and Collagen Membrane in Surgical Manage- an interpositional material in surgical management of OSMF and ment of Oral Submucous Fibrosis. Int J Prev Clin Dent Res also (1) to assess and compare the mouth opening achieved in 2017;4(3):210-217. both groups of patient; (2) the improvement in flexibility of buccal mucosa in both groups; (3) oral pain and burning sensation on Source of support: Nil intake of spicy food; (4) the rapidity in epithelialization of graft at the intraoral wound site. Conflict of interest: None Materials and methods: Thirty patients were randomly divided into 15 patients each in groups I and II respectively. Group I INTRODUCTION patients received BFP as the interpositioning material, whereas group II received xenogenous collagen membrane after bilateral In 1952, Schwartz described five Indian women from excision of bands. Group I was compared with group II postop- Kenya with a condition of the oral mucosa including the eratively for mouth opening up to 6 months follow-up. -

Buccal Fat Pad Augmentation for Facial Rejuvenation Steven R
TITLE PAGE (AUTHORS, CORRESPONDING AUTHOR, AFFILIATION, FINANCIAL DISCLOSURE, Buccal Fat Pad Augmentation for Facial Rejuvenation 1,2 Steven R. Cohen MD, FACS 1 Emily Fireman MS4 2 Sierra Hewett 1,2 Ahmad Saad MD From the Division of Plastic Surgery, University of California, San Diego1 and FACES+ Plastic Surgery, Dermatology, Skin 2 and Laser Center, San Diego, California Corresponding Author: Steven R. Cohen MD 4510 Executive Drive Suite 200 San Diego, CA 92121; [email protected]; 858-453-7224 Disclosures: Dr. Cohen consults for Tulip Medical, Inc. and Millenium Medical Technologies, Inc. and is a paid investigator, consultant and minor shareholder for Cytori, Inc. MANUSCRIPT (TITLE, MANUSCRIPT TEXT, REFERENCES, LEGENDS) Buccal Fat Pad Augmentation for Facial Rejuvenation ABSTRACT Background: The buccal space and its fat pad (BFP) is a valuable, overlooked target in facial rejuvenation procedures. We identified a specific group of patients who have normal or prominent malar projection in the presence of atrophy of the buccal fat pad, with or without prominent gonial angles. Materials and Methods: Eight of 24 prospectively studied patients (BIOMED IRB), who had fat grafts and facelifts received an average of 2.7 ml of fat transferred into the buccal space. Immediate visual correction of the buccal depression was noted. No overcorrection was carried out. Results: None of the 8 patients suffered an adverse event from trans-oral buccal space fat grafting. Persistent facial volume in this area has been noted up to 24 months after treatment. Conclusions: In patients with buccal fat pad atrophy, fat grafting into the buccal space can be safely performed through an intraoral approach. -

BUCCAL FAT PAD FLAP Johan Fagan
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY BUCCAL FAT PAD FLAP Johan Fagan The buccal fat pad flap is an axial flap and may be used to fill small-to-medium sized soft tissue and bony defects in the palate, superior and inferior alveoli and buccal mucosa. It is often encountered as it bulges into the surgical field during surgery in the pterygomandibular region. Relevant Anatomy Buccal fat pad The buccal fat pad (Figure 1) is an en- capsulated, mass of specialized fatty tissue, the volume of which varies throughout life. Figure 2: MRI (axial view) illustrating the It is distinct from subcutaneous fat (Figure anatomical relationship of the buccal fat 2). It fills the deep tissue spaces and acts as pad to masseter and buccinator muscles gliding pads when masticatory and mime- tic muscles contract, and cushions impor- The buccal fat pad has a body and four tant structures from forces generated by processes. The body is located behind the muscle contraction. zygomatic arch. The body is divided into 3 lobes – anterior, intermediate and poste- rior, in accordance with the structure of the lobar envelopes, the ligaments and the Temporalis feeding vessels. The anterior lobe is loca- Buccal fat pad ted below the zygoma, and extends to the front of the buccinator, maxilla and the Masseter deep space of the quadrate muscle of the Buccinator upper lip and zygomaticus major muscle. Parotid duct The canine muscle originates from the Masseter infraorbital foramen and passes through the medial part of the anterior lobe. The Adapted from http://en.wikipedia.org/wiki/Buccal_fat_pad parotid duct passes through the posterior Figure 1: Buccal fat pad part, and the anterior facial vein passes through the anteroinferior margin. -

Use of Buccal Fat Pad and Collagen in the Surgical Management of Oral Submucous Fibrosis- a Pilot Study
40 Indian Journal of Contemporary Dentistry,Type: January-June Original Research 2020, Vol.8, No.1 Use of Buccal Fat Pad and Collagen in the Surgical Management of Oral Submucous Fibrosis- A Pilot Study Nilesh Bhanawat1, Vikash Ranjan2, Soumendu Bikash Maiti3, Priyank Rai4 1Reader, Department of Oral & Maxillofacial Surgery, Pacific Dental College & Research Centre, Udaipur, Raj, 2Associate Professor, 3Senior Lecture, Department of Oral Medicine and Radiology, Divya Jyoti Collge Of Dental Sciences And Research, Modinagar, 4Senior Lecture , Department of Oral & Maxillofacial Surgery, Pacific Dental College & Research Centre, Udaipur, Raj Abstract Background: Various surgical modalities have been tried in surgical management of submucous fibrosis, but each has its own limitations. Aims & Objectives: To assess the efficacy of buccal pad of fat covered with collagen membrane as a grafting material in the management of Oral Submucous fibrosis. Materials & Method: Seven Grade II and Grade III Submucous fibrosis patients were randomly included in the sample population. Surgical release of the fibrous band and closure of the surgical defect with buccal pad of fat covered with collagen membrane as the interposition material was done. The post operative mouth opening and healing and patient comfort was statistically analzed using SPSS v.20. Results: The mean pre operative mouth opening of the patients was 15.29 + 4.751 mm. The mean post operative mouth opening at the end of 6 month is 30.86+2.268mm. Conclusion: With minimal post operative complication and avoidance of a secondary donor site morbidity, this technique can be safely employed in the management of OSMF patients. Keywords: Oral Submucous Fibrosis; Buccal Pad of Fat; collagen Membrane. -

Versatility and Importance of Bichat's Fat Pad in Dentistry
JCDP Juan FD Montero et al 10.5005/jp-journals-10024-2352 CASE REPORT Versatility and Importance of Bichat’s Fat Pad in Dentistry: Case Reports of Its Use in Occlusal Trauma 1Juan FD Montero, 2Humberto CM de Souza, 3Mariana S Martins, 4Miguel N Oliveira, 5César AM Benfatti 6Ricardo de Souza Magini ABSTRACT Clinical significance: Application of Bichat’s fat and its removal should be evaluated, being an alternative in patients who con- Introduction: The knowledge of the anatomy surrounding stantly undergo mucosal injury during masticatory function. Bichat’s fat pad, as well as its clinical applications, is essential to indicate and to safely perform its removal. This surgery is Keywords: Adipose tissue, Bichat’s fat pad, Oral lesions. indicated not only for esthetic purposes, but also for functional How to cite this article: Montero JFD, de Souza HCM, reasons. When used properly, Bichat’s fat pad is composed of Martins MS, Oliveira MN, Benfatti CAM, de Souza Magini R. stem cells that have a similar phenotype to adipose stem cells, Versatility and Importance of Bichat’s Fat Pad in Dentistry: Case useful in the treatment of pathologies and/or complications, such Reports of Its Use in Occlusal Trauma. J Contemp Dent Pract as maxillary sinus membrane perforation, oroantral/oronasal 2018;19(7):888-894. communications, peri-implantitis, ulcers, fibrosis of the oral mucosa, soft tissue reconstruction, among others. Due to its Source of support: Nil location, it is prone to suffer clinically significant pathologies, Conflict of interest: None as well as constant trauma. Aim: The aim of this study is to report two clinical cases and subsequent follow-ups, where bichectomy was performed to INTRODUCTION avoid dental trauma to mucosal tissues during the masticatory The adipose body of the cheek, also known as Bichat’s fat function.