Physical Constraints and Forces Involved in Phagocytosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Membrane Tension Buffering by Caveolae: a Role in Cancer?
Cancer and Metastasis Reviews (2020) 39:505–517 https://doi.org/10.1007/s10555-020-09899-2 Membrane tension buffering by caveolae: a role in cancer? Vibha Singh1 & Christophe Lamaze1 Published online: 30 May 2020 # Springer Science+Business Media, LLC, part of Springer Nature 2020 Abstract Caveolae are bulb-like invaginations made up of two essential structural proteins, caveolin-1 and cavins, which are abundantly present at the plasma membrane of vertebrate cells. Since their discovery more than 60 years ago, the function of caveolae has been mired in controversy. The last decade has seen the characterization of new caveolae components and regulators together with the discovery of additional cellular functions that have shed new light on these enigmatic structures. Early on, caveolae and/ or caveolin-1 have been involved in the regulation of several parameters associated with cancer progression such as cell migration, metastasis, angiogenesis, or cell growth. These studies have revealed that caveolin-1 and more recently cavin-1 have a dual role with either a negative or a positive effect on most of these parameters. The recent discovery that caveolae can act as mechanosensors has sparked an array of new studies that have addressed the mechanobiology of caveolae in various cellular functions. This review summarizes the current knowledge on caveolae and their role in cancer development through their activity in membrane tension buffering. We propose that the role of caveolae in cancer has to be revisited through their response to the mechanical forces encountered by cancer cells during tumor mass development. Keywords Caveolae . Cancer . Mechanosensing . Mechanotransdcution . Membrane tension . -

Table 1 Gene Name Increased Or Decreased in LTD
Table_1 gene_name increased or decreased in LTD protein_id description keep_supernatant keep_pellet comparison sample hit_annotation_methodpvalue fdr hit hit_annotation 2010300C02RIK increased E9Q3M9 Protein 2010300C02Rik OS=Mus musculus GN=2010300C02Rik PE=1 SV=1 TRUE TRUE NMDA - control pellet fdrtool 0,074667 0,584087 FALSE trend 2310035C23RIK|KIAA1468increased A0A087WSS1|E9QM90|Q148V7|Q148V7-2 Protein 2310035C23Rik OS=Mus musculus GN=2310035C23Rik PE=1 SV=1|Protein 2310035C23Rik OS=Mus musculusTRUE GN=2310035C23Rik PE=1 SV=2|LisH FALSE domain NMDAand HEAT - control repeat-containing protein KIAA1468 supernatant OS=Mus musculus GN=Kiaa1468 fdrtoolPE=1 SV=1|Isoform 0,080056 2 of 0,589077LisH domain FALSEand HEAT trend repeat-containing protein KIAA1468 OS=Mus musculus GN=Kiaa1468 ABR increased E9PUE7|Q5SSL4|Q5SSL4-2|Q5SSL4-3|Q5SSL4-4 Active breakpoint cluster region-related protein OS=Mus musculus GN=Abr PE=1 SV=1|Isoform 2 of Active breakpointTRUE cluster region-related protein TRUE OS=Mus musculus NMDA GN=Abr|Isoform - control 3 of Active breakpoint supernatant cluster region-related protein OS=Mus fdrtool musculus 0,08128 GN=Abr|Isoform 0,592743 4 of Active FALSE breakpoint trend cluster region-related protein OS=Mus musculus GN=Abr ADAM22 increased D3YUP9|Q9R1V6|Q9R1V6-10|Q9R1V6-11|Q9R1V6-12|Q9R1V6-13|Q9R1V6-14|Q9R1V6-15|Q9R1V6-17|Q9R1V6-4|Q9R1V6-5|Q9R1V6-6|Q9R1V6-7|Q9R1V6-8Disintegrin and metalloproteinase domain-containing protein 22 OS=Mus musculus GN=Adam22 PE=1 SV=1|DisintegrinFALSE and metalloproteinase domain-containing TRUE -

UCSD MOLECULE PAGES Doi:10.6072/H0.MP.A002549.01 Volume 1, Issue 2, 2012 Copyright UC Press, All Rights Reserved
UCSD MOLECULE PAGES doi:10.6072/H0.MP.A002549.01 Volume 1, Issue 2, 2012 Copyright UC Press, All rights reserved. Review Article Open Access WAVE2 Tadaomi Takenawa1, Shiro Suetsugu2, Daisuke Yamazaki3, Shusaku Kurisu1 WASP family verprolin-homologous protein 2 (WAVE2, also called WASF2) was originally identified by its sequence similarity at the carboxy-terminal VCA (verprolin, cofilin/central, acidic) domain with Wiskott-Aldrich syndrome protein (WASP) and N-WASP (neural WASP). In mammals, WAVE2 is ubiquitously expressed, and its two paralogs, WAVE1 (also called suppressor of cAMP receptor 1, SCAR1) and WAVE3, are predominantly expressed in the brain. The VCA domain of WASP and WAVE family proteins can activate the actin-related protein 2/3 (Arp2/3) complex, a major actin nucleator in cells. Proteins that can activate the Arp2/3 complex are now collectively known as nucleation-promoting factors (NPFs), and the WASP and WAVE families are a founding class of NPFs. The WAVE family has an amino-terminal WAVE homology domain (WHD domain, also called the SCAR homology domain, SHD) followed by the proline-rich region that interacts with various Src-homology 3 (SH3) domain proteins. The VCA domain located at the C-terminus. WAVE2, like WAVE1 and WAVE3, constitutively forms a huge heteropentameric protein complex (the WANP complex), binding through its WHD domain with Abi-1 (or its paralogs, Abi-2 and Abi-3), HSPC300 (also called Brick1), Nap1 (also called Hem-2 and NCKAP1), Sra1 (also called p140Sra1 and CYFIP1; its paralog is PIR121 or CYFIP2). The WANP complex is recruited to the plasma membrane by cooperative action of activated Rac GTPases and acidic phosphoinositides. -

An Animal Model with a Cardiomyocyte-Specific Deletion of Estrogen Receptor Alpha: Functional, Metabolic, and Differential Netwo
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2014 An animal model with a cardiomyocyte-specific deletion of estrogen receptor alpha: Functional, metabolic, and differential network analysis Sriram Devanathan Washington University School of Medicine in St. Louis Timothy Whitehead Washington University School of Medicine in St. Louis George G. Schweitzer Washington University School of Medicine in St. Louis Nicole Fettig Washington University School of Medicine in St. Louis Attila Kovacs Washington University School of Medicine in St. Louis See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Devanathan, Sriram; Whitehead, Timothy; Schweitzer, George G.; Fettig, Nicole; Kovacs, Attila; Korach, Kenneth S.; Finck, Brian N.; and Shoghi, Kooresh I., ,"An animal model with a cardiomyocyte-specific deletion of estrogen receptor alpha: Functional, metabolic, and differential network analysis." PLoS One.9,7. e101900. (2014). https://digitalcommons.wustl.edu/open_access_pubs/3326 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Authors Sriram Devanathan, Timothy Whitehead, George G. Schweitzer, Nicole Fettig, Attila Kovacs, Kenneth S. Korach, Brian N. Finck, and Kooresh I. Shoghi This open access publication is available at Digital Commons@Becker: https://digitalcommons.wustl.edu/open_access_pubs/3326 An Animal Model with a Cardiomyocyte-Specific Deletion of Estrogen Receptor Alpha: Functional, Metabolic, and Differential Network Analysis Sriram Devanathan1, Timothy Whitehead1, George G. Schweitzer2, Nicole Fettig1, Attila Kovacs3, Kenneth S. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -
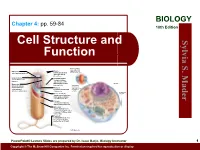
Cell Structure and Function
BIOLOGY Chapter 4: pp. 59-84 10th Edition Cell Structure and S. Sylvia Function Plasma membrane: outer surface that Ribosome: Fimbriae: regulates entrance site of protein synthesis hairlike bristles that and exit of molecules allow adhesion to Mader the surfaces Inclusion body: Conjugation pilus: stored nutrients for elongated, hollow later use appendage used for DNA transfer to other Nucleus: Mesosome: bacterial cells plasma membrane Cytoskeleton: maintains cell that folds into the Nucleoid: shape and assists cytoplasm and location of the bacterial movement of increases surface area chromosome cell parts: Endoplasmic Plasma membrane: reticulum: sheath around cytoplasm that regulates entrance and exit of molecules Cell wall: covering that supports, shapes, and protects cell Glycocalyx: gel-like coating outside cell wall; if compact, called a capsule; if diffuse, called a slime layer Flagellum: rotating filament present in some bacteria that pushes the cell forward *not in plant cells PowerPoint® Lecture Slides are prepared by Dr. Isaac Barjis, Biology Instructor 1 Copyright © The McGraw Hill Companies Inc. Permission required for reproduction or display Outline Cellular Level of Organization Cell theory Cell size Prokaryotic Cells Eukaryotic Cells Organelles Nucleus and Ribosome Endomembrane System Other Vesicles and Vacuoles Energy related organelles Cytoskeleton Centrioles, Cilia, and Flagella 2 Cell Theory Detailed study of the cell began in the 1830s A unifying concept in biology Originated from the work of biologists Schleiden and Schwann in 1838-9 States that: All organisms are composed of cells German botanist Matthais Schleiden in 1838 German zoologist Theodor Schwann in 1839 All cells come only from preexisting cells German physician Rudolph Virchow in 1850’s Cells are the smallest structural and functional unit of organisms 3 Organisms and Cells Copyright © The McGraw-Hill Companies, Inc. -

Serum Albumin OS=Homo Sapiens
Protein Name Cluster of Glial fibrillary acidic protein OS=Homo sapiens GN=GFAP PE=1 SV=1 (P14136) Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 Cluster of Isoform 3 of Plectin OS=Homo sapiens GN=PLEC (Q15149-3) Cluster of Hemoglobin subunit beta OS=Homo sapiens GN=HBB PE=1 SV=2 (P68871) Vimentin OS=Homo sapiens GN=VIM PE=1 SV=4 Cluster of Tubulin beta-3 chain OS=Homo sapiens GN=TUBB3 PE=1 SV=2 (Q13509) Cluster of Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 (P60709) Cluster of Tubulin alpha-1B chain OS=Homo sapiens GN=TUBA1B PE=1 SV=1 (P68363) Cluster of Isoform 2 of Spectrin alpha chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTAN1 (Q13813-2) Hemoglobin subunit alpha OS=Homo sapiens GN=HBA1 PE=1 SV=2 Cluster of Spectrin beta chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTBN1 PE=1 SV=2 (Q01082) Cluster of Pyruvate kinase isozymes M1/M2 OS=Homo sapiens GN=PKM PE=1 SV=4 (P14618) Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 Clathrin heavy chain 1 OS=Homo sapiens GN=CLTC PE=1 SV=5 Filamin-A OS=Homo sapiens GN=FLNA PE=1 SV=4 Cytoplasmic dynein 1 heavy chain 1 OS=Homo sapiens GN=DYNC1H1 PE=1 SV=5 Cluster of ATPase, Na+/K+ transporting, alpha 2 (+) polypeptide OS=Homo sapiens GN=ATP1A2 PE=3 SV=1 (B1AKY9) Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 Dihydropyrimidinase-related protein 2 OS=Homo sapiens GN=DPYSL2 PE=1 SV=1 Cluster of Alpha-actinin-1 OS=Homo sapiens GN=ACTN1 PE=1 SV=2 (P12814) 60 kDa heat shock protein, mitochondrial OS=Homo -

Phagocytosis Dynamics Depends on Target Shape
Biophysical Journal Volume 105 September 2013 1143–1150 1143 Phagocytosis Dynamics Depends on Target Shape Debjani Paul,†‡6* Sarra Achouri,†6 Young-Zoon Yoon,† Jurgen Herre,§ Clare E. Bryant,{ and Pietro Cicuta† † ‡ Cavendish Laboratory, University of Cambridge, Cambridge, United Kingdom; Department of Biosciences and Bioengineering, § { Indian Institute of Technology Bombay, Powai, Mumbai, India; Department of Medicine and Department of Veterinary Medicine, University of Cambridge, Cambridge, United Kingdom ABSTRACT A complete understanding of phagocytosis requires insight into both its biochemical and physical aspects. One of the ways to explore the physical mechanism of phagocytosis is to probe whether and how the target properties (e.g., size, shape, surface states, stiffness, etc.) affect their uptake. Here we report an imaging-based method to explore phagocytosis kinetics, which is compatible with real-time imaging and can be used to validate existing reports using fixed and stained cells. We measure single-event engulfment time from a large number of phagocytosis events to compare how size and shape of targets determine their engulfment. The data shows an increase in the average engulfment time for increased target size, for spherical particles. The uptake time data on nonspherical particles confirms that target shape plays a more dominant role than target size for phagocytosis: Ellipsoids with an eccentricity of 0.954 and much smaller surface areas than spheres were taken up five times more slowly than spherical targets. INTRODUCTION Macrophages can engulf a wide variety of targets, such as the degradation of phagosomal contents and the induction invading pathogens, dead cells, foreign airborne particles of the appropriate immune responses. -

Actin Nucleator Spire 1 Is a Regulator of Ectoplasmic Specialization in the Testis Qing Wen1,Nanli1,Xiangxiao 1,2,Wing-Yeelui3, Darren S
Wen et al. Cell Death and Disease (2018) 9:208 DOI 10.1038/s41419-017-0201-6 Cell Death & Disease ARTICLE Open Access Actin nucleator Spire 1 is a regulator of ectoplasmic specialization in the testis Qing Wen1,NanLi1,XiangXiao 1,2,Wing-yeeLui3, Darren S. Chu1, Chris K. C. Wong4, Qingquan Lian5,RenshanGe5, Will M. Lee3, Bruno Silvestrini6 and C. Yan Cheng 1 Abstract Germ cell differentiation during the epithelial cycle of spermatogenesis is accompanied by extensive remodeling at the Sertoli cell–cell and Sertoli cell–spermatid interface to accommodate the transport of preleptotene spermatocytes and developing spermatids across the blood–testis barrier (BTB) and the adluminal compartment of the seminiferous epithelium, respectively. The unique cell junction in the testis is the actin-rich ectoplasmic specialization (ES) designated basal ES at the Sertoli cell–cell interface, and the apical ES at the Sertoli–spermatid interface. Since ES dynamics (i.e., disassembly, reassembly and stabilization) are supported by actin microfilaments, which rapidly converts between their bundled and unbundled/branched configuration to confer plasticity to the ES, it is logical to speculate that actin nucleation proteins play a crucial role to ES dynamics. Herein, we reported findings that Spire 1, an actin nucleator known to polymerize actins into long stretches of linear microfilaments in cells, is an important regulator of ES dynamics. Its knockdown by RNAi in Sertoli cells cultured in vitro was found to impede the Sertoli cell tight junction (TJ)-permeability barrier through changes in the organization of F-actin across Sertoli cell cytosol. Unexpectedly, Spire 1 knockdown also perturbed microtubule (MT) organization in Sertoli cells cultured in vitro. -

NIH Public Access Author Manuscript Mucosal Immunol
NIH Public Access Author Manuscript Mucosal Immunol. Author manuscript; available in PMC 2014 March 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Mucosal Immunol. 2013 March ; 6(2): 379–392. doi:10.1038/mi.2012.81. Molecular Organization of the Mucins and Glycocalyx Underlying Mucus Transport Over Mucosal Surfaces of the Airways Mehmet Kesimer*,‡,▫, Camille Ehre*, Kimberly A. Burns*, C. William Davis*,†, John K. Sheehan*,‡, and Raymond J. Pickles*,§ *Cystic Fibrosis/Pulmonary Research and Treatment Center, University of North Carolina, Chapel Hill, NC 27599-7248 ‡Department of Biochemistry and Biophysics, University of North Carolina, Chapel Hill, NC 27599-7248 †Department of Cell and Molecular Physiology, University of North Carolina, Chapel Hill, NC 27599-7248 §Department of Microbiology and Immunology, University of North Carolina, Chapel Hill, NC 27599-7248 Abstract Mucus, with its burden of inspired particulates, and pathogens, is cleared from mucosal surfaces of the airways by cilia beating within the periciliary layer (PCL). The PCL is held to be ‘watery’ and free of mucus by thixotropic-like forces arising from beating cilia. With radii of gyration ~250 nm, however, polymeric mucins should reptate readily into the PCL, so we assessed the glycocalyx for barrier functions. The PCL stained negative for MUC5AC and MUC5B, but it was positive for keratan sulfate, a glycosaminoglycan commonly associated with glycoconjugates. Shotgun proteomics showed keratan sulfate-rich fractions from mucus containing abundant tethered mucins, MUC1, MUC4, and MUC16, but no proteoglycans. Immuno-histology by light and electron microscopy localized MUC1 to microvilli, MUC4 and MUC20 to cilia, and MUC16 to goblet cells. -

Chloroplast Incorporation and Long-Term Photosynthetic Performance Through the Life Cycle in Laboratory Cultures of Elysia Timid
Chloroplast incorporation and long-term photosynthetic performance through the life cycle in laboratory cultures of Elysia timida (Sacoglossa, Heterobranchia) Schmitt et al. Schmitt et al. Frontiers in Zoology 2014, 11:5 http://www.frontiersinzoology.com/content/11/1/5 Schmitt et al. Frontiers in Zoology 2014, 11:5 http://www.frontiersinzoology.com/content/11/1/5 RESEARCH Open Access Chloroplast incorporation and long-term photosynthetic performance through the life cycle in laboratory cultures of Elysia timida (Sacoglossa, Heterobranchia) Valerie Schmitt1,2, Katharina Händeler2, Susanne Gunkel2, Marie-Line Escande3, Diedrik Menzel4, Sven B Gould2, William F Martin2 and Heike Wägele1* Abstract Introduction: The Mediterranean sacoglossan Elysia timida is one of the few sea slug species with the ability to sequester chloroplasts from its food algae and to subsequently store them in a functional state in the digestive gland cells for more than a month, during which time the plastids retain high photosynthetic activity (= long-term retention). Adult E. timida have been described to feed on the unicellular alga Acetabularia acetabulum in their natural environment. The suitability of E. timida as a laboratory model culture system including its food source was studied. Results: In contrast to the literature reporting that juvenile E. timida feed on Cladophora dalmatica first, and later on switch to the adult diet A. acetabulum, the juveniles in this study fed directly on A. acetabulum (young, non-calcified stalks); they did not feed on the various Cladophora spp. (collected from the sea or laboratory culture) offered. This could possibly hint to cryptic speciation with no clear morphological differences, but incipient ecological differentiation. -

The Role of the Actin Cytoskeleton During Muscle Development In
THE ROLE OF THE ACTIN CYTOSKELETON DURING MUSCLE DEVELOPMENT IN DROSOPHILA AND MOUSE by Shannon Faye Yu A Dissertation Presented to the Faculty of the Louis V. Gerstner, Jr. Graduate School of the Biomedical Sciences in Partial Fulfillment of the Requirements of the Degree of Doctor of Philosophy New York, NY Oct, 2013 Mary K. Baylies, PhD! Date Dissertation Mentor Copyright by Shannon F. Yu 2013 ABSTRACT The actin cytoskeleton is essential for many processes within a developing organism. Unsurprisingly, actin and its regulators underpin many of the critical steps in the formation and function of muscle tissue. These include cell division during the specification of muscle progenitors, myoblast fusion, muscle elongation and attachment, and muscle maturation, including sarcomere assembly. Analysis in Drosophila has focused on regulators of actin polymerization particularly during myoblast fusion, and the conservation of many of the actin regulators required for muscle development has not yet been tested. In addition, dynamic actin processes also require the depolymerization of existing actin fibers to replenish the pool of actin monomers available for polymerization. Despite this, the role of actin depolymerization has not been described in depth in Drosophila or mammalian muscle development. ! Here, we first examine the role of the actin depolymerization factor Twinstar (Tsr) in muscle development in Drosophila. We show that Twinstar, the sole Drosophila member of the ADF/cofilin family of actin depolymerization proteins, is expressed in muscle where it is essential for development. tsr mutant embryos displayed a number of muscle defects, including muscle loss and muscle misattachment. Further, regulators of Tsr, including a Tsr-inactivating kinase, Center divider, a Tsr-activating phosphatase, Slingshot and a synergistic partner in depolymerization, Flare, are also required for embryonic muscle development.