An Electromyographic Analysis of Lateral Raise Variations and Frontal Raise in Competitive Bodybuilders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Structure and Function of Breathing
CHAPTERCONTENTS The structure-function continuum 1 Multiple Influences: biomechanical, biochemical and psychological 1 The structure and Homeostasis and heterostasis 2 OBJECTIVE AND METHODS 4 function of breathing NORMAL BREATHING 5 Respiratory benefits 5 Leon Chaitow The upper airway 5 Dinah Bradley Thenose 5 The oropharynx 13 The larynx 13 Pathological states affecting the airways 13 Normal posture and other structural THE STRUCTURE-FUNCTION considerations 14 Further structural considerations 15 CONTINUUM Kapandji's model 16 Nowhere in the body is the axiom of structure Structural features of breathing 16 governing function more apparent than in its Lung volumes and capacities 19 relation to respiration. This is also a region in Fascla and resplrstory function 20 which prolonged modifications of function - Thoracic spine and ribs 21 Discs 22 such as the inappropriate breathing pattern dis- Structural features of the ribs 22 played during hyperventilation - inevitably intercostal musculature 23 induce structural changes, for example involving Structural features of the sternum 23 Posterior thorax 23 accessory breathing muscles as well as the tho- Palpation landmarks 23 racic articulations. Ultimately, the self-perpetuat- NEURAL REGULATION OF BREATHING 24 ing cycle of functional change creating structural Chemical control of breathing 25 modification leading to reinforced dysfunctional Voluntary control of breathing 25 tendencies can become complete, from The autonomic nervous system 26 whichever direction dysfunction arrives, for Sympathetic division 27 Parasympathetic division 27 example: structural adaptations can prevent NANC system 28 normal breathing function, and abnormal breath- THE MUSCLES OF RESPIRATION 30 ing function ensures continued structural adap- Additional soft tissue influences and tational stresses leading to decompensation. -

Extended Insertion of Teres Minor Muscle: a Rare Case Report
Eur J Anat, 16 (3): 224-225 (2012) CASE REPORT Extended insertion of teres minor muscle: a rare case report Monica Jain, Lovesh Shukla, Dalbir Kaur Maharaja Agrasen Medical College, Agroha-125047, Hisar, Haryana, India SUMMARY upwards and laterally, and gets inserted on the lowest of the three impressions on the greater Teres minor is one of the muscles of the shoul- tubercle of the humerus and fuses with the der joint along with subscapularis, supraspina- capsule of the shoulder joint along with other tus and infraspinatus forming rotator cuff. muscles forming the rotator cuff. It is inner- Variations of teres minor are relatively uncom- vated by the posterior branch of the axillary mon. A unique and extended insertion of this nerve. It stabilizes the humerus by holding muscle is being reported in the present case. the humeral head in the glenoid cavity of the Knowledge of the anatomy of this muscle is scapula, a and causes lateral rotation of the important to avoid injury to the axillary nerve arm (Johnson, 2008). Variations of teres and posterior circumflex humeral vessels while minor are relatively uncommon and have been surgically approaching the shoulder joint and occasionally reported by various authors inserting portals of the arthroscope in a poste- (Bergman et al., 2006). rior approach to the shoulder joint. Key words: Teres minor – Rotator cuff – CASE REPORT Shoulder joint – Capsule of shoulder joint – Humerus – Surgical neck of humerus During routine dissection of the shoulder region of upper limb of an approximately 50 year-old male cadaver for undergraduate teach- INTRODUCTION ing and training, a unique and extended inser- tion of the teres minor muscle was found on the Teres minor is a one of the short scapular right side. -

Tricepsterrific
ACE-SPONSORED RESEARCH TricepsTerrific omen from all walks of life struggle to avoid the dreaded flabby, jiggly arms—and they often turn to personal trainers and fitness pros for help. “Guys always want to get rid of their bellies, while women always By Brittany Boehler,W B.S., seem to want to tone their triceps,” says John P. Porcari, Ph.D., John Porcari, Ph.D., an exercise physiologist Dennis Kline, M.S., with the University of Wisconsin and former C. Russell Hendrix, Ph.D., and personal trainer. Carl Foster, Ph.D., with Mark Anders But as with most clients, their time is constantly being gobbled up by work and family obligations, leaving little extra time for regular exercise. They want results—and fast! With that in mind, the This study was funded solely by the American Council on Exercise, the nation’s Work- American Council on Exercise (ACE). out Watchdog, sponsored comprehensive research to determine which exercises are most effec- tive—and efficient—for targeting the triceps. Armed with this new research, you’ll be able to better guide your clients in their efforts to tone and strengthen their triceps. TheTo determine Study the efficacy of the eight most common triceps exercises, ACE enlisted a team of exercise scientists from the University of Wiscon- sin/La Crosse Exercise and Health Program. Led www.acefitness.org pg. 1 ACE-SPONSORED RESEARCH by John Porcari, Ph.D., and Brittany Boehler, B.S., the exercise to ensure proper muscle recovery. Subjects lifted research team recruited 15 healthy female subjects, ages 70 percent of their previously determined 1 RM for the 20 to 24, from the local La Crosse community. -

Active Release Techniques Spine Level 2
Active Release Techniques Spine Level 2 Dates of program- Montvale, NJ February 18-21, 2021 Colorado Springs, CO March 4-7, 2021 Orlando, FL June 10-13, 2021 Chicago, IL September 30 – October 3, 2021 Total Hours: 24 Summary: Active Release Techniques® Spine Level 2 offers intense training in 75 manual treatment protocols of the cervical, thoracic, and lumbar spine. ART® treatment utilizes manual techniques to move tissues and joints while under tension. The system allows for relative motion between the tissues and articulations. This seminar emphasizes the manipulation of the neuromusculoskeletal system to diagnose and correct alterations in tissue texture, tension, movement, and function between tissues. Evaluation and treatment occur simultaneously. Learning Outcomes: 1. By the end of the seminar, learners will be able to correctly identify (palpate) 75 facial seams of soft-tissue structures within the spine. 2. By the end of the seminars, learners will be able to correctly state the muscle actions of two adjacent spinal muscles. 3. By the end of the seminar, learners will be able to effectively recognize common symptom patterns of spinal neuromuscular injuries and disorders. 4. By the end of the seminar, learners will correctly identify the structure treated and associated concentric and eccentric muscle actions via video presentations. 5. By the end of the seminar, the learner will correctly move the muscle from its shortened position to elongated position using two-hand placement techniques. 6. By the end of the seminar, the learner can successfully differentiate between healthy and unhealthy tissue utilizing hands-on palpation techniques. 7. By the end of the seminar, the learner will proficiently palpate 75 anatomical soft-tissue structures within the spine, using an appropriate tension, depth, and motion to properly perform the treatment protocol. -

Anatomical, Clinical, and Electrodiagnostic Features of Radial Neuropathies
Anatomical, Clinical, and Electrodiagnostic Features of Radial Neuropathies a, b Leo H. Wang, MD, PhD *, Michael D. Weiss, MD KEYWORDS Radial Posterior interosseous Neuropathy Electrodiagnostic study KEY POINTS The radial nerve subserves the extensor compartment of the arm. Radial nerve lesions are common because of the length and winding course of the nerve. The radial nerve is in direct contact with bone at the midpoint and distal third of the humerus, and therefore most vulnerable to compression or contusion from fractures. Electrodiagnostic studies are useful to localize and characterize the injury as axonal or demyelinating. Radial neuropathies at the midhumeral shaft tend to have good prognosis. INTRODUCTION The radial nerve is the principal nerve in the upper extremity that subserves the extensor compartments of the arm. It has a long and winding course rendering it vulnerable to injury. Radial neuropathies are commonly a consequence of acute trau- matic injury and only rarely caused by entrapment in the absence of such an injury. This article reviews the anatomy of the radial nerve, common sites of injury and their presentation, and the electrodiagnostic approach to localizing the lesion. ANATOMY OF THE RADIAL NERVE Course of the Radial Nerve The radial nerve subserves the extensors of the arms and fingers and the sensory nerves of the extensor surface of the arm.1–3 Because it serves the sensory and motor Disclosures: Dr Wang has no relevant disclosures. Dr Weiss is a consultant for CSL-Behring and a speaker for Grifols Inc. and Walgreens. He has research support from the Northeast ALS Consortium and ALS Therapy Alliance. -
Monday: Back, Biceps, Forearms, Traps & Abs Wednesday
THE TOOLS YOU NEED TO BUILD THE BODY YOU WANT® Store Workouts Diet Plans Expert Guides Videos Tools BULLDOZER TRAINING 3 DAY WORKOUT SPLIT 3 day Bulldozer Training muscle building split. Combines rest-pause sets with progressive Main Goal: Build Muscle Time Per Workout: 30-45 Mins resistance. Workouts are shorter but more Training Level: Intermediate Equipment: Barbell, Bodyweight, intense. Program Duration: 8 Weeks Dumbbells, EZ Bar, Machines Link to Workout: https://www.muscleandstrength.com/ Days Per Week: 3 Days Author: Steve Shaw workouts/bulldozer-training-3-day-workout-split Monday: Back, Biceps, Forearms, Traps & Abs Exercise Mini Sets Rep Goal Rest Deadlift: Perform as many rest-paused singles as you (safely) can within 10 Mins. Use a weight you could easily perform a 10 rep set with. Rest as needed. When you can perform 15 reps, add weight the next time you deadlift. Barbell Row 5 25 30 / 30 / 45 / 45 Wide Grip Pull Up 5 35 30 / 30 / 30 / 30 Standing Dumbbell Curl 4 25 30 / 30 / 30 EZ Bar Preacher Curl 4 25 30 / 30 / 30 Seated Barbell Wrist Curl 4 35 30 / 30 / 30 Barbell Shrug 5 35 30 / 30 / 30 / 30 Preferred Abs Exercise(s): I recommend using at least one weighted exercise (e.g. Weighted Sit Ups or Cable Crunches). Rest Periods: 30 / 30 / 45 / 45 notates rest periods between each set. Take 30 Secs after the 1st set, 30 Secs after the 2nd set, 45 Secs after the 3rd set, etc. After the final set, rest, and move on to the next exercise. -

Bilateral Sternalis Muscles Were Observed During Dissection of the Thoraco-Abdominal Region of a Male Cadaver
Case Reports Ahmed F. Ibrahim, MSc, MD, Saeed A. Makarem, MSc. PhD, Hassem H. Darwish, MBBCh. ABSTRACT Bilateral sternalis muscles were observed during dissection of the thoraco-abdominal region of a male cadaver. A full description of the muscles, as well as their attachments and innervations were reported. A brief review of the existing literature, regarding the nomenclature, incidence, attachments, innervations and clinical relevance of the sternalis muscle, is also presented. Neurosciences 2005; Vol. 10 (2): 171-173 he importance of continuing to record and Case Report. A well defined sternalis muscle Tdiscuss anatomical anomalies was addressed (Figures 1 & 2) was found, bilaterally, during recently1 in light of technical advances and dissection of the thoraco-abdominal region of a interventional methods of diagnosis and treatment. male cadaver in the Department of Anatomy, The sternalis muscle is a small supernumerary College of Medicine, King Saud University, Riyadh, muscle located in the anterior thoracic region, Kingdom of Saudi Arabia. Both muscles were superficial to the sternum and the sternocostal covered by superficial fascia, located superficial to fascicles of the pectoralis major muscle.2 In the the corresponding sternocostal portion of pectoralis literature, sternalis muscle is called "a normal major and separated from it by pectoral fascia. The anatomic variant"3 and "a well-known variation",4 left sternalis was 19 cm long and 3 cm wide at its although in most textbooks of anatomy, it is broadest part. Its upper end formed a tendon insufficiently mentioned. Yet, clinicians are continuous with that of the sternal head of left surprisingly unaware of this common variation. -

M1 – Muscled Arm
M1 – Muscled Arm See diagram on next page 1. tendinous junction 38. brachial artery 2. dorsal interosseous muscles of hand 39. humerus 3. radial nerve 40. lateral epicondyle of humerus 4. radial artery 41. tendon of flexor carpi radialis muscle 5. extensor retinaculum 42. median nerve 6. abductor pollicis brevis muscle 43. flexor retinaculum 7. extensor carpi radialis brevis muscle 44. tendon of palmaris longus muscle 8. extensor carpi radialis longus muscle 45. common palmar digital nerves of 9. brachioradialis muscle median nerve 10. brachialis muscle 46. flexor pollicis brevis muscle 11. deltoid muscle 47. adductor pollicis muscle 12. supraspinatus muscle 48. lumbrical muscles of hand 13. scapular spine 49. tendon of flexor digitorium 14. trapezius muscle superficialis muscle 15. infraspinatus muscle 50. superficial transverse metacarpal 16. latissimus dorsi muscle ligament 17. teres major muscle 51. common palmar digital arteries 18. teres minor muscle 52. digital synovial sheath 19. triangular space 53. tendon of flexor digitorum profundus 20. long head of triceps brachii muscle muscle 21. lateral head of triceps brachii muscle 54. annular part of fibrous tendon 22. tendon of triceps brachii muscle sheaths 23. ulnar nerve 55. proper palmar digital nerves of ulnar 24. anconeus muscle nerve 25. medial epicondyle of humerus 56. cruciform part of fibrous tendon 26. olecranon process of ulna sheaths 27. flexor carpi ulnaris muscle 57. superficial palmar arch 28. extensor digitorum muscle of hand 58. abductor digiti minimi muscle of hand 29. extensor carpi ulnaris muscle 59. opponens digiti minimi muscle of 30. tendon of extensor digitorium muscle hand of hand 60. superficial branch of ulnar nerve 31. -

How to Administer Intramuscular and Subcutaneous Vaccines to Adults
How to Administer Intramuscular and Subcutaneous Vaccine Injections to Adults Intramuscular (IM) Injections Administer these vaccines via IM route • Haemophilus influenzae type b (Hib) • Hepatitis A (HepA) • Hepatitis B (HepB) • Human papillomavirus (HPV) acromion process (bony prominence above deltoid) • Influenza vaccine, injectable (IIV) • level of armpit • Influenza vaccine, recombinant (RIV3; RIV4) • Meningococcal conjugate (MenACWY) IM injection site • Meningococcal serogroup B (MenB) (shaded area = deltoid muscle) • Pneumococcal conjugate (PCV13) • Pneumococcal polysaccharide (PPSV23) – elbow may also be given Subcut • Polio (IPV) – may also be given Subcut • Tetanus, diphtheria (Td), or with pertussis (Tdap) • Zoster, recombinant (RZV; Shingrix) 90° angle Injection site skin Give in the central and thickest portion of the deltoid muscle – above the level of the armpit and approximately subcutaneous tissue 2–3 fingerbreadths (~2") below the acromion process. See the diagram. To avoid causing an injury, do not inject muscle too high (near the acromion process) or too low. Needle size Note: A ⅝" needle is sufficient in adults weighing less than 130 lbs (<60 kg) for IM injection in the deltoid muscle only if the subcutane- 22–25 gauge, 1–1½" needle (see note at right) ous tissue is not bunched and the injection is made at a 90° angle; Needle insertion a 1" needle is sufficient in adults weighing 130–152 lbs (60–70 kg); a 1–1½" needle is recommended in women weighing 153–200 lbs • Use a needle long enough to reach deep into the muscle. (70–90 kg) and men weighing 153–260 lbs (70–118 kg); a 1½" needle • Insert the needle at a 90° angle to the skin with a quick is recommended in women weighing more than 200 lbs (91 kg) or thrust. -

MRI Patterns of Shoulder Denervation: a Way to Make It Easy
MRI Patterns Of Shoulder Denervation: A Way To Make It Easy Poster No.: C-2059 Congress: ECR 2018 Type: Educational Exhibit Authors: E. Rossetto1, P. Schvartzman2, V. N. Alarcon2, M. E. Scherer2, D. M. Cecchi3, F. M. Olivera Plata4; 1Buenos Aires, Capital Federal/ AR, 2Buenos Aires/AR, 3Capital Federal, Buenos Aires/AR, 4Ciudad Autonoma de Buenos Aires/AR Keywords: Musculoskeletal joint, Musculoskeletal soft tissue, Neuroradiology peripheral nerve, MR, Education, eLearning, Edema, Inflammation, Education and training DOI: 10.1594/ecr2018/C-2059 Any information contained in this pdf file is automatically generated from digital material submitted to EPOS by third parties in the form of scientific presentations. References to any names, marks, products, or services of third parties or hypertext links to third- party sites or information are provided solely as a convenience to you and do not in any way constitute or imply ECR's endorsement, sponsorship or recommendation of the third party, information, product or service. ECR is not responsible for the content of these pages and does not make any representations regarding the content or accuracy of material in this file. As per copyright regulations, any unauthorised use of the material or parts thereof as well as commercial reproduction or multiple distribution by any traditional or electronically based reproduction/publication method ist strictly prohibited. You agree to defend, indemnify, and hold ECR harmless from and against any and all claims, damages, costs, and expenses, including attorneys' fees, arising from or related to your use of these pages. Please note: Links to movies, ppt slideshows and any other multimedia files are not available in the pdf version of presentations. -
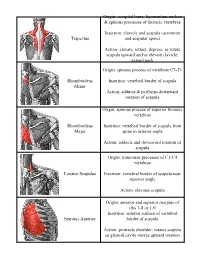
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

The Laminated Nature of the Pectoralis Major Muscle and the Redefinition of the Inframammary Fold Clinical Implications in Aesthetic and Reconstructive Breast Surgery
The Laminated Nature of the Pectoralis Major Muscle and the Redefinition of the Inframammary Fold Clinical Implications in Aesthetic and Reconstructive Breast Surgery Melvin M. Maclin II, MDa,*, Olivier A. Deigni, MD, MPHb, Bradley P. Bengtson, MDc KEYWORDS Pectoralis major muscle Inframammary fold Subpectoral augmentation Breast augmentation Breast reconstruction Acellular dermal matrix Breast inflection points Chest wall anatomy KEY POINTS The inframammary fold (IMF) is a critical landmark and aesthetic structure in breast surgery, yet it is poorly understood. The skin envelope is considered a separate entity from the chest wall; however, its surgical manip- ulation is not independent of chest wall anatomy. The pectoralis major muscle is a key structure in both cosmetic and reconstructive surgery, and its structure and performance are related to its inferior costal origins. A better understanding of the relationship of the IMF, pectoralis, and chest wall anatomy can offer improved outcomes in breast surgery. INTRODUCTION intimately aware of its relationship to the chest The breast is appreciated aesthetically and clini- wall and the breast soft tissues. Both are able to cally for its shape, projection, and volume. Multiple achieve outstanding outcomes; however, the au- techniques have evolved over the years to modify, thors present an alternative appreciation of the enhance, or recreate the breast mound. To this pectoralis and its relationship to the breast. The end surgical techniques have evolved to manipu- authors liken the comparison to the tale retold by late the breast skin envelope, soft tissues, and John Saxe of the 6 blind wise men and the chest wall anatomy, with and without prosthetic elephant (Fig.