Boards Fodder Vaccines in Dermatology by Caroline A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Letters to the Editor
Lepr Rev (1994) 65, 282-285 Letters to the Editor CONCOMITANT OCCURRENCE OF LEPROSY, CUTANEOUS TUBERCULOSIS AND PULMONARY TUBERCULOSIS-A CASE REPORT Sir, We report a leprosy patient also suffering from both cutaneous and pulmonary tuberculosis, a concomitant occurrence that has not previously been reported in the literature available to us. We report here a case of such rare combination. Though both the diseases are caused by mycobacter iae, no true antagonism exists to stop coexistence. The concomitant occurrence of leprosy and pulmonary tuberculosis has been well documented in the literature, 1,2 but the association of leprosy and cutaneous tuberculosis has rarely been reported.3,4,5 A 23-year-old male presented complaining of an erythematous lesion around the left orbit that Figure 1. An erythematous, oedematous lesion on the left sideof the forehead and infraorbital area, that almost encircles the orbit. 282 Letters to the Editor 283 Figure 2. Multiple ulcers in linear fashion with undermined edges and marginal hyperpigmentation on the left side of the neck. had continued for I month and multiple ulcerations with a discharge of pus on the left side of the neck for 15 days; ulcerations followed rupturing of the swelling in the neck. The swelling was of I!-months' duration, mildly painful and was gradually increasing in size. There was a history of a rise of temperature each evening and of significantweight loss. He had not been treated for leprosy and/or tuberculosis. Cutaneous examination revealed a well-defined erythematous plaque around the left orbit (Figure I). There were multiple ulcers in linear fa shion over the left side of the neck with undermined edges and hyperpigmented borders (Figure 2). -

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -

Vaccinia Belongs to a Family of Viruses That Is Closely Related to the Smallpox Virus
VACCINIA INFECTION What is it? Vaccinia belongs to a family of viruses that is closely related to the smallpox virus. Because of the similarities between the smallpox and vaccinia viruses, the vaccinia virus is used in the smallpox vaccine. When this virus is used as a vaccine, it allows our immune systems to develop immunity against smallpox. The smallpox vaccine does not actually contain smallpox virus and cannot cause smallpox. Vaccination usually prevents smallpox infection for at least ten years. The vaccinia vaccine against smallpox was used to successfully eradicate smallpox from the human population. More recently, this virus has also become of interest due to concerns about smallpox being used as an agent of bioterrorism. How is the virus spread? Vaccinia can be spread by touching the vaccination site before it has fully healed or by touching clothing or bandages that have been contaminated with the live virus during vaccination. In this manner, vaccinia can spread to other parts of the body and to other individuals. It cannot be spread through the air. What are the symptoms of vaccinia? Vaccinia virus symptoms are similar to smallpox, but milder. Vaccinia may cause rash, fever, headache and body aches. In certain individuals, such as those with weak immune systems, the symptoms can be more severe. What are the potential side effects of the vaccinia vaccine for smallpox? Normal reactions are mild and go away without any treatment.These include: Soreness and redness in the arm where the vaccine was given Slightly swollen, sore glands in the armpits Low grade fever One in approximately three people will feel badly enough to miss school, work or recreational activities Trouble sleeping Serious reactions are not very common but can occur in about 1,000 in every 1 million people who are vaccinated for the first time. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Pattern of Cutaneous Tuberculosis Among Children and Adolescent
Bangladesh Med Res Counc Bull 2012; 38: 94-97 Pattern of cutaneous tuberculosis among children and adolescent Sultana A1, Bhuiyan MSI1, Haque A2, Bashar A3, Islam MT4, Rahman MM5 1Dept. of Dermatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, 2Dept. of Public health and informatics, BSMMU, Dhaka, 3SK Hospital, Mymensingh Medical College, Mymensingh, 4Dept. of Physical Medicine and Rehabilitation, BSMMU, Dhaka, 5Dept. of Dermatology, National Medical College, Dhaka. Email: [email protected] Abstract Cutaneous tuberculosis is one of the most subtle and difficult diagnoses for dermatologists practicing in developing countries. It has widely varied manifestations and it is important to know the spectrum of manifestations in children and adolescent. Sixty cases (age<19 years) of cutaneous tuberculosis were included in this one period study. The diagnosis was based on clinical examination, tuberculin reaction, histopathology, and response to antitubercular therapy. Histopahology revealed 38.3% had skin tuberculosis and 61.7% had diseases other than tuberculosis. Among 23 histopathologically proved cutaneous tuberculosis, 47.8% had scrofuloderma, 34.8% had lupus vulgaris and 17.4% had tuberculosis verrucosa cutis (TVC). Most common site for scrofuloderma lesions was neck and that for lupus vulgaris and TVC was lower limb. Cutaneous tuberculosis in children continues to be an important cause of morbidity, there is a high likelihood of internal involvement, especially in patients with scrofuloderma. A search is required for more sensitive, economic diagnostic tools. Introduction of Child Health (BICH) and Institute of Diseases of Tuberculosis (TB), an ancient disease has affected Chest and Hospital (IDCH) from January to humankind for more than 4,000 years1 and its December 2010. -
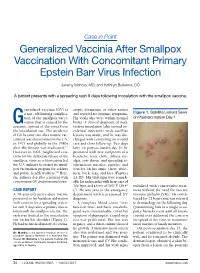
Generalized Vaccinia After Smallpox Vaccination with Concomitant Primary Epstein Barr Virus Infection
Case in Point Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection Jeremy Mandia, MD; and Kathryn Buikema, DO A patient presents with a spreading rash 9 days following inoculation with the smallpox vaccine. eneralized vaccinia (GV) is atopic dermatitis, or other rashes a rare, self-limiting complica- and reported no systemic symptoms. Figure 1. Satellite Lesions Seen tion of the smallpox vacci- His vitals also were within normal on Postvaccination Day 1 Gnation that is caused by the limits. A clinical diagnosis of inad- systemic spread of the virus from vertent inoculation (also termed ac- the inoculation site. The incidence cidental infection) with satellite of GV became rare after routine vac- lesions was made, and he was dis- cination was discontinued in the U.S. charged with counseling on wound in 1971 and globally in the 1980s care and close follow-up. Two days after the disease was eradicated.1,2 later, on postvaccination day 11, he However in 2002, heightened con- presented with new symptoms of a cerns for the deliberate release of the headache, fever, chills, diffuse my- smallpox virus as a bioweapon led algia, sore throat, and spreading er- the U.S. military to restart its small- ythematous macules, papules, and pox vaccination program for soldiers vesicles on his arms, chest, abdo- and public health workers.3,4 Here, men, back, legs, and face (Figures the authors describe a patient with 2A-2D). His vital signs were remark- concomitant GV and mononucleosis. able for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º stabilized with conservative treat- CASE REPORT C). -

ORIGINAL ARTICLE a Clinical and Histopathological Study of Lichenoid Eruption of Skin in Two Tertiary Care Hospitals of Dhaka
ORIGINAL ARTICLE A Clinical and Histopathological study of Lichenoid Eruption of Skin in Two Tertiary Care Hospitals of Dhaka. Khaled A1, Banu SG 2, Kamal M 3, Manzoor J 4, Nasir TA 5 Introduction studies from other countries. Skin diseases manifested by lichenoid eruption, With this background, this present study was is common in our country. Patients usually undertaken to know the clinical and attend the skin disease clinic in advanced stage histopathological pattern of lichenoid eruption, of disease because of improper treatment due to age and sex distribution of the diseases and to difficulties in differentiation of myriads of well assess the clinical diagnostic accuracy by established diseases which present as lichenoid histopathology. eruption. When we call a clinical eruption lichenoid, we Materials and Method usually mean it resembles lichen planus1, the A total of 134 cases were included in this study prototype of this group of disease. The term and these cases were collected from lichenoid used clinically to describe a flat Bangabandhu Sheikh Mujib Medical University topped, shiny papular eruption resembling 2 (Jan 2003 to Feb 2005) and Apollo Hospitals lichen planus. Histopathologically these Dhaka (Oct 2006 to May 2008), both of these are diseases show lichenoid tissue reaction. The large tertiary care hospitals in Dhaka. Biopsy lichenoid tissue reaction is characterized by specimen from patients of all age group having epidermal basal cell damage that is intimately lichenoid eruption was included in this study. associated with massive infiltration of T cells in 3 Detailed clinical history including age, sex, upper dermis. distribution of lesions, presence of itching, The spectrum of clinical diseases related to exacerbating factors, drug history, family history lichenoid tissue reaction is wider and usually and any systemic manifestation were noted. -

Cidofovir Activity Against Poxvirus Infections
Viruses 2010 , 2, 2803-2830; doi:10.3390/v2122803 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Cidofovir Activity against Poxvirus Infections Graciela Andrei * and Robert Snoeck Laboratory of Virology and Chemotherapy, Rega Institute for Medical Research, KULeuven, Minderboredersstraat 10, B-3000 Leuven, Belgium; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-16-337372; Fax: +32-16-337340. Received: 10 November 2010; in revised form: 9 December 2010 / Accepted: 10 December 2010 / Published: 22 December 2010 Abstract: Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, HPMPC] is an acyclic nucleoside analog approved since 1996 for clinical use in the treatment of cytomegalovirus (CMV) retinitis in AIDS patients. Cidofovir (CDV) has broad-spectrum activity against DNA viruses, including herpes-, adeno-, polyoma-, papilloma- and poxviruses. Among poxviruses, cidofovir has shown in vitro activity against orthopox [vaccinia, variola (smallpox), cowpox, monkeypox, camelpox, ectromelia], molluscipox [molluscum contagiosum] and parapox [orf] viruses. The anti-poxvirus activity of cidofovir in vivo has been shown in different models of infection when the compound was administered either intraperitoneal, intranasal (aerosolized) or topically. In humans, cidofovir has been successfully used for the treatment of recalcitrant molluscum contagiosum virus and orf virus in immunocompromised patients. CDV remains a reference compound against poxviruses and holds potential for the therapy and short-term prophylaxis of not only orthopox- but also parapox- and molluscipoxvirus infections. Keywords: cidofovir; poxviruses; acyclic nucleoside analog 1. Introduction The antiviral activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC, cidofovir, CDV) (Figure 1) against human cytomegalovirus (HCMV) and other DNA viruses was first Viruses 2010 , 2 2804 reported in 1986 [1]. -

Clinical Study of Nail Changes in Papulosquamous Disorders
Original Research Article Clinical study of Nail changes in papulosquamous disorders Vishal Wali1, Trivedi Rahul Prasad2* 1Associate Professor, 2Post Graduate, Department of Dermatology, Venereology and Leprology, Mahadevappa Rampure Medical College, Sedam Road, Kalaburagi (Gulbarga) 585105, Karnataka, INDIA. Email: [email protected] Abstract Background: Nail disorders comprise ~10% of all dermatologic conditions. Nail disorders include those abnormalities that effect any portion of the nail unit. The nail unit includes the plate, matrix, bed, proximal and lateral folds, hyponychium, and some definitions include the underlying distal phalanx. These structures may be effected by heredity, skin disorders, infections, systemic disease, and aging process, internal and external medications, physical and environmental agents, trauma, and tumours, both benign and malignant. The main contributors being papulosquamous disorder. Nail changes in papulosquamous disorder have been inadequately discussed and only limited studies are present. This study aims to throw some light about frequency of nail involvement in papulosquamous disorders and its various patterns. Methodology: This is the descriptive study. It was conducted in department of DVL of Mahadevappa Rampura Medical college and Basaveshwar teaching and general hospital, Kalaburagi from July 2019 to Feb 2021. General, systemic and dermatological examinations were done. Nails were examined in detail. Special investigations like skin biopsy and potassium hydroxide (KOH) mount was done in relevant cases. Results: There were 50 cases of papulosquamous disorder. The most common papulosquamous disorder was psoriasis, followed by lichen planus and PRP. Out of these the most common nail change was pitting (81%) and lest common was dorsal pterygium (%) Conclusion: Nail being an important appendage affecting various dermatosis and acts as window in diagnosis. -

Case for Diagnosis* Caso Para Diagnóstico*
RevABDV81N5.qxd 09.11.06 09:42 Page 490 490 Qual o seu diagnóstico? Caso para diagnóstico* Case for diagnosis* Rodrigo Pereira Duquia1 Hiram Larangeira de Almeida Jr2 Ernani Siegmann Duvelius3 Manfred Wolter4 HISTÓRIA DA DOENÇA Paciente do sexo feminino, de 76 anos, há lesões liquenóides dorsais, solicitação do teste de dois anos apresentou linfadenopatias na região Mantoux e exames laboratoriais, com a suspeita de cervical (Figura 1) e axilar esquerda. Após um ano scrofuloderma e líquen scrofulosorum. Nessa oca- iniciou fistulização e drenagem de material esbran- sião a paciente apresentava-se em mau estado geral quiçado das lesões, emagrecimento e aparecimen- com febre persistente, emagrecimento importante to de lesões anulares liquenóides com centro atró- e astenia. fico na região dorsal (Figura 2), levemente prurigi- O Mantoux foi fortemente reator, 24mm, velo- nosas. Há seis meses realizou biópsia da região cer- cidade de sedimentação globular de 96mm, a cultura vical, que foi inconclusiva. Posteriormente foi enca- da secreção cervical foi negativa, e PCR foi positiva minhada para avaliação dermatológica, sendo reali- para Mycobacterium tuberculosis. zada coleta de material da região cervical, enviado O exame histopatológico das lesões do dorso então para cultura e reação em cadeia pela polime- revelou espongiose focal na epiderme com alguns rase (PCR). Além disso, realizaram-se biópsia das queratinócitos necróticos, apresentando na derme FIGURA 1: Lesões eritematosas com crosta hemática recobrindo as FIGURA 2: Lesões liquenóides, anulares, com atrofia central na fístulas na região cervical esquerda região dorsal. No detalhe à direita, a atrofia central e os bordos papulosos e anulares ficam mais evidentes Recebido em 22.02.2006. -

Medical Management of Biological Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES FORT DETRICK FREDERICK, MARYLAND Emergency Response Numbers National Response Center: 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Helpline: 1-410-436-4484 or (Edgewood Ops Center – for military use) DSN 584-4484 USAMRIID’s Emergency Response Line: 1-888-872-7443 CDC'S Emergency Response Line: 1-770-488-7100 Handbook Download Site An Adobe Acrobat Reader (pdf file) version of this handbook can be downloaded from the internet at the following url: http://www.usamriid.army.mil USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 Lead Editor Lt Col Jon B. Woods, MC, USAF Contributing Editors CAPT Robert G. Darling, MC, USN LTC Zygmunt F. Dembek, MS, USAR Lt Col Bridget K. Carr, MSC, USAF COL Ted J. Cieslak, MC, USA LCDR James V. Lawler, MC, USN MAJ Anthony C. Littrell, MC, USA LTC Mark G. Kortepeter, MC, USA LTC Nelson W. Rebert, MS, USA LTC Scott A. Stanek, MC, USA COL James W. Martin, MC, USA Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 PREFACE TO THE SIXTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination.