(ACIP) General Best Guidance for Immunization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
FROM DNA to BEER Date Lesson Plan: Acquired and Passive Immunity Class Period
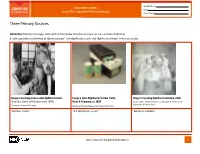
Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, ca. 1898 Courtesy The Historical Medical Library of The College of Physicians of Philadelphia Courtesy Library of Congress Courtesy National Museum of American History “diphtheria toxin”: “anti-diphtheritic serum”: “diphtheria antitoxin”: http://www.nlm.nih.gov/fromdnatobeer 1 Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources 2. Describe or draw how the three images may be related. http://www.nlm.nih.gov/fromdnatobeer 2 FROM DNA TO BEER Lesson Plan: Acquired and Passive Immunity Teacher’s Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, -

Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP)
Morbidity and Mortality Weekly Report Recommendations and Reports February 8, 2002 / Vol. 51 / No. RR-2 General Recommendations on Immunization Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) INSIDE: Continuing Education Examination Centers for Disease Control and Prevention SAFER • HEALTHIER • PEOPLETM MMWR CONTENTS The MMWR series of publications is published by the Introduction ......................................................................... 1 Epidemiology Program Office, Centers for Disease Timing and Spacing of Immunobiologics .............................. 2 General Principles for Vaccine Scheduling ......................... 2 Control and Prevention (CDC), U.S. Department of Spacing of Multiple Doses of the Same Antigen ................ 2 Health and Human Services, Atlanta, GA 30333. Simultaneous Administration ............................................ 4 Nonsimultaneous Administration ...................................... 5 Spacing of Antibody-Containing Products and Vaccines ..... 6 SUGGESTED CITATION Interchangeability of Vaccines from Different Manufacturers 8 Centers for Disease Control and Prevention. General Lapsed Vaccination Schedule ............................................ 8 recommendations on immunization: recom- Unknown or Uncertain Vaccination Status ......................... 8 mendations of the Advisory Committee on Contraindications and Precautions ....................................... 8 Immunization Practices and the -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

Prospecting for an HIV Vaccine D
Brett-Major et al. Tropical Diseases, Travel Medicine and Vaccines (2017) 3:6 DOI 10.1186/s40794-017-0050-4 REVIEW Open Access Prospecting for an HIV vaccine D. M. Brett-Major1,2*, T. A. Crowell1,2 and N. L. Michael1 Abstract Human immunodeficiency virus (HIV) sets several challenges for the development of a preventative HIV vaccine. Predictable, protective natural immunity against HIV does not occur and so unlike most other diseases for which vaccines exist, there are few guideposts from natural infection. Nonetheless, six vaccine efficacy trials have occurred. One in particular, the Thai trial called RV144, showed partial protective efficacy and potential ways ahead to a better vaccine approach. This coupled with other lessons from studies of acute infections as well as an increasingly complex knowledge of HIV-related vaccine immunology bring hope that a vaccine solution might be reached for this pervasive and deadly pandemic. Keywords: Human immunodeficiency virus vaccine protective efficacy immunology review Background Why not already Human immunodeficiency virus (HIV) disease remains A reasonable person new to the global conversation one of the greatest threats to global public health. about HIV might ask, if an HIV vaccine is so critical and According to the World Health Organization (WHO), in the pandemic known for three decades, why do we not 2014 over one million people died from HIV, nearly already have an HIV vaccine? There is no simple answer thirty-seven million people had chronic infection and to this question, though an easy one is that people do two million people newly acquired infections [1]. Of not develop natural, protective immunity to HIV infec- those persons known to be HIV infected, only 35% tion and disease. -

The Model of “Informed Refusal” for Vaccination: How to Fight Against Anti-Vaccinationist Misinformation Without Disregarding the Principle of Self-Determination
Communication The Model of “Informed Refusal” for Vaccination: How to Fight against Anti-Vaccinationist Misinformation without Disregarding the Principle of Self-Determination Stefano D’Errico 1, Emanuela Turillazzi 2, Martina Zanon 1, Rocco Valerio Viola 3, Paola Frati 3,4 and Vittorio Fineschi 3,4,* 1 Department of Surgery, Medicine and Health, University of Trieste, 34149 Trieste, Italy; [email protected] (S.D.); [email protected] (M.Z.) 2 Department of Surgical Pathology, Medical, Molecular and Critical Area, Institute of Legal Medicine, University of Pisa, 56126 Pisa, Italy; [email protected] 3 Department of Anatomical, Histological, Forensic and Orthopaedic Sciences, Sapienza University of Rome, Viale Regina Elena 336, 00161 Rome, Italy; [email protected] (R.V.V.); [email protected] (P.F.) 4 IRCCS (Istituto di Ricerca e Cura a Carattere Scientifico) Neuromed Mediterranean Neurological Institute, Via Atinense 18, 86077 Pozzilli, Italy * Correspondence: [email protected]; Tel.: +39 06 49912722 Abstract: Vaccines are arguably a public health success story as well as an incredibly cost-effective medical resource. Despite this, worldwide concerns about their safety are growing, with the risk of Citation: D’Errico, S.; Turillazzi, E.; increased morbidity and mortality in vaccine-preventable diseases because of vaccine refusal. The Zanon, M.; Viola, R.V.; Frati, P.; global political trend in developed countries is to increasingly reduce mandates and the compulsory Fineschi, V. The Model of “Informed nature of vaccination programs. This is due to strong opposition from anti-vaccination movements Refusal” For Vaccination: How to and groups. While these have existed since the beginnings of vaccinology, they have recently gained Fight Against Anti-Vaccinationist a strong foothold through massive exploitation of the media and especially the internet. -

The Study of Thimerosal and Autism
The Study of Thimerosal and Autism Documentation and Codebook for the Child Vaccination Histories File: Cambridge, MA Lexington, MA Hadley, MA Bethesda, MD September 3, 2010 Chicago, IL Prepared for Frank DeStefano National Immunization Program Centers for Disease Control and Prevention Atlanta, GA 30329 Prepared by Cristofer Price Yeqin He Abt Associates Inc. Suite 800 North 4550 Montgomery Avenue Bethesda, MD 20814-3343 This documentation was prepared by Cristofer Price and Yeqin He of Abt Associates Inc. for the Immunization Safety Office (ISO) of the Centers for Disease Control and Prevention (CDC) Atlanta, GA 30333. Questions about the documentation, substantive questions, or technical issues regarding the data file should be directed to the CDC ISO, MS D26, 1600 Clifton Road, Atlanta, Georgia 30333 (404-639-8256). Suggested Citation for Study of Thimerosal and Autism Public Use Data Set: Price, C.S., He, Y. (2010) The Study of Thimerosal and Autism: Data Set Documentation. Bethesda MD: Abt Associates, Inc; Prepared for the National Immunization Program Centers for Disease Control and Prevention Atlanta, GA Abt Associates Inc. 1 Documentation and Codebook for the Child Vaccination Histories File 1. Introduction to the Child Vaccination Histories File............................................... 2 2. File Formats and Variable Descriptions................................................................... 4 1. Introduction to the Child Vaccination Histories File The Child Vaccination Histories File contains the data that were used to construct the measures of early childhood (postnatal) exposure to ethylmercury from thimerosal- containing vaccines and immune globulin preparations received by the study children during the age range spanning birth to 20 months. The Child Vaccination Histories File is provided with the public use data in order to make the calculation of exposure amounts transparent, and so that analysts have the potential to calculate alternative measures of exposure1. -

Investigator's Brochure: Vaccinia Immune Globulin
Use of Vaccinia Immune Globulin (VIG) For Treatment of vaccinia vaccine complications and for prophylaxis of unvaccinated individuals exposed to Orthopoxviruses responsive to VIG Investigational New Drug (IND) Proposal TABLE OF CONTENTS: 1. Introduction............................................................Page 2 2. Effects in Humans..................................................Pages 3 - 4 3. Nonclinical Studies.................................................Pages 5- 6 4. Rationale and Objectives.........................................Page 7 5. Study Design... .......................................................Page 8 6. Additional Information - Selected References..........Pages 9 - 15 Page -1- INVESTIGATOR’S BROCHURE INTRODUCTION This Investigational New Drug Application is being filed in order to make existing stocks of Vaccinia Immune Globulin available for treatment of complications of vaccinia vaccination or exposure to vaccinia or related pox viruses. Vaccinia Immune Globulin (VIG) (Human) is an isotonic sterile solution of the immunoglobulin fraction of plasma from persons vaccinated with vaccinia vaccine. The current lot, 0448A101A, was released as a licensed product by Baxter Healthcare Corporation in 1995. In 1998 a slight discoloration was noted in this product and the FDA placed a hold on its release. Consequently, VIG can no longer be entered into interstate commerce under terms of the approved license and must therefore revert to IND (investigational new drug) status. CDC follows the June 22, 2001 Recommendations of the Immunization Practices Advisory Committee (ACIP) for Vaccinia (Smallpox) Vaccine. Since 1983 CDC has provided vaccinia vaccine for laboratory workers directly involved with smallpox or closely related orthopox viruses (e.g., monkeypox and vaccinia). Due to clinical trials involving recombinant vaccinia virus vaccines, health-care workers (e.g., physicians and nurses) may now be exposed to vaccinia and recombinant vaccinia viruses and may be considered for vaccinia vaccination. -

COVID-19: About Vaccines This Guidance Provides Basic Information Only
COVID-19: About Vaccines This guidance provides basic information only. It is not intended to take the place of medical advice, diagnosis or treatment, legal advice or legal requirements. • Please check the Government of New Brunswick’s COVID-19 website regularly for updates to this document. list of symptoms, other guidance documents, Directives and other information. COVID-19 Vaccines: Overview Representing a turning point in our fight against COVID-19, Health Canada has authorized the Pfizer-BioNTech mRNA vaccine. More vaccines will likely be authorized in the near future.1 What you should know: • Health Canada only approves a vaccine if it is supported by very robust scientific data and evidence. • After approval, Health Canada and the Public Health Agency of Canada continue to monitor the ongoing safety and effectiveness of all approved vaccines. • Canadians will have easy access to detailed information on the vaccine and the evidence behind the vaccine approval process through the Government of Canada's website. • The benefits of vaccination greatly outweigh the risks, and many more illnesses and deaths would occur without vaccines. Vaccines prevent illness and disease and save lives and livelihoods. Mass vaccination will protect people's lives and help Canada recover from the COVID-19 pandemic. After more than a decade of research and development on mRNA vaccines, this vaccine is the first mRNA vaccine approved for use in humans. To date, mRNA has been successfully used in cancer treatments, and research into its value for vaccinations has been ongoing for over ten years. 1 Soon after original publication of this document, Health Canada authorized the Moderna mRNA vaccine. -

Diphtheria. In: Epidemiology and Prevention of Vaccine
Diphtheria Anna M. Acosta, MD; Pedro L. Moro, MD, MPH; Susan Hariri, PhD; and Tejpratap S.P. Tiwari, MD Diphtheria is an acute, bacterial disease caused by toxin- producing strains of Corynebacterium diphtheriae. The name Diphtheria of the disease is derived from the Greek diphthera, meaning ● Described by Hippocrates in ‘leather hide.’ The disease was described in the 5th century 5th century BCE BCE by Hippocrates, and epidemics were described in the ● Epidemics described in 6th century AD by Aetius. The bacterium was first observed 6th century in diphtheritic membranes by Edwin Klebs in 1883 and cultivated by Friedrich Löffler in 1884. Beginning in the early ● Bacterium first observed in 1900s, prophylaxis was attempted with combinations of toxin 1883 and cultivated in 1884 and antitoxin. Diphtheria toxoid was developed in the early ● Diphtheria toxoid developed 7 1920s but was not widely used until the early 1930s. It was in 1920s incorporated with tetanus toxoid and pertussis vaccine and became routinely used in the 1940s. Corynebacterium diphtheria Corynebacterium diphtheriae ● Aerobic gram-positive bacillus C. diphtheriae is an aerobic, gram-positive bacillus. ● Toxin production occurs Toxin production (toxigenicity) occurs only when the when bacillus is infected bacillus is itself infected (lysogenized) by specific viruses by corynebacteriophages (corynebacteriophages) carrying the genetic information for carrying tox gene the toxin (tox gene). Diphtheria toxin causes the local and systemic manifestations of diphtheria. ● Four biotypes: gravis, intermedius, mitis, and belfanti C. diphtheriae has four biotypes: gravis, intermedius, mitis, ● All isolates should be tested and belfanti. All biotypes can become toxigenic and cause for toxigenicity severe disease. -

COVID-19 Vaccines Under Development | Piedmont Healthcare
COVID-19 Vaccines Under Development: Where are we? Dr. Grace Gowda Director, International Biomedical Regulatory Sciences [email protected] No Relevant Financial Relationship with Piedmont Healthcare or Companies Discussed in the Presentation Agenda • Definitions • Emergency Use Authorizations • Vaccine Development & Clinical Trials • COVID 19 Vaccine Development • Genetic Vaccines • Moderna Vaccine • Pfizer/BioNTech Vaccine • Viral Vector Vaccines • Protein Vaccines • Inactivated or Attenuated Vaccines 3 Definitions What is a Vaccine? • A vaccine stimulates the immune system to produce antibodies, exactly like it would if you were exposed to the disease. [CDC] • A vaccine is a substance that helps in protecting against certain diseases. Vaccines contain a dead or weakened version of a microbe. It helps your immune system recognize and destroy the living microbe during a future infection. [Web MD] https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html 4 Definitions What is COVID-19? • It is a coronavirus disease identified in 2019. Symptoms usually appear from 2-14 days after exposure to the virus. • People with the following symptoms may have COVID-19 • Fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, diarrhea What is an Emergency Use Authorization (EUA)? • It is a legal mechanism in the US that allows FDA during a public health emergency to facilitate the availability -

CME INDIA COVID-19 Vaccination Protocol 2021 July Updat
COVID-19 VACCINATION PROTOCOL JULY 21 CME INDIA CME 1 CME INDIA CME INDIA COVID-19 Vaccination Protocol First published: 29th April 2021. Last Updated: 6th July 2021. With the rapid rise in Covid-19 cases in INDIA, CME INDIA has now compiled its own vaccination protocol with inputs from key medical experts. The goal of this document is to help the medical community in managing the current Covid-19 situation and provide insight into the COVID-19 Vaccination Program in India. This document will be updated from time to time, so please check www.cmeindia.in and CME INDIA Downloads section regularly. Alternatively, you can choose to navigate to the various key sections of CME INDIA: Explore CME INDIA Repository Examine CME INDIA Case Study Read History Today in Medicine Register for Future CMEs View CME INDIA Downloads 2 CME INDIA 3 CME INDIA Basic Framework By: Dr. Akash Singh, MD (Med) MSc (Diabetes) Consultant Physician and Diabetologist Spandan Multi Speciality Hospital, Vadodara. Dr. S. K. Gupta, MD (Med), FICP, CFM(France) Clinical Asst. Professor GS Medical College, CCSU, Uttar Pradesh India. Visiting Consultant, Max Super Specialty Hospital, New Delhi. Dr. Kiran Shah, Consultant Physician MBBS, MD, Spandan Multi Speciality Hospital Vadodara. Edited By: Dr. N.K. Singh, MD, FICP, Diabetologist Physician, Dhanbad, Editor - cmeindia.in. Advisor and Reviewer: Dr. Awadhesh Kumar Singh, Consultant Endocrinologist, G. D. Hospital & Diabetes Institute, Kolkata, West Bengal. Invitee Reviewer: Dr. Banshi Saboo, National President, RSSDI, Ahmedabad. Dr. Mangesh Tiwaskar, Consultant Physician & Diabetologist, Mumbai, Hon. General Secretary, API. Reviewer: Dr. R. Rajasekar, MD, FICP, FACP (USA), FRCP (Glasgow), FRCP (Ireland), Consultant Physician & Diabetologist Heart & Diabetes Therapy Centre, Kumbakonam, Tamil Nadu. -

Mass Vaccination: When and Why
CTMI (2006) 304:1–16 Mass Vaccination: When and Why D. L. Heymann (u)·R.B.Aylward World Health Organization, 1211 Geneva 27, Switzerland [email protected] 1 The Concept of Mass Vaccination ............................. 2 2 Mass vaccination in the Twentieth Century—Smallpox Eradication .... 3 3 Routine Immunization and Mass Vaccination Today— A Necessary Alliance ..................................... 4 4 Preventing Emerging Outbreaks ............................. 5 4.1 Meningitis............................................. 6 4.2 Influenza.............................................. 7 4.3 YellowFever............................................ 8 4.4 DisplacedPersons....................................... 8 4.5 ThreatofDeliberatelyCausedOutbreaks....................... 9 5 Mass Vaccination to Accelerate Disease Control .................. 10 5.1 NewVaccineIntroduction.................................. 11 5.2 Eradication............................................ 12 5.3 MortalityReduction...................................... 13 5.3.1 Measles............................................... 13 5.3.2 MaternalandNeonatalTetanus.............................. 13 6 Mass Vaccination in the Twenty-First Century .................... 13 References .................................................. 14 Abstract With increased demand for smallpox vaccination during the nineteenth cen- tury, vaccination days—early mass vaccination campaigns—were conducted over time-limited periods to rapidly and efficiently protect maximum numbers of suscepti- ble persons.