Diphtheria. In: Epidemiology and Prevention of Vaccine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ID 2 | Issue No: 4.1 | Issue Date: 29.10.14 | Page: 1 of 24 © Crown Copyright 2014 Identification of Corynebacterium Species
UK Standards for Microbiology Investigations Identification of Corynebacterium species Issued by the Standards Unit, Microbiology Services, PHE Bacteriology – Identification | ID 2 | Issue no: 4.1 | Issue date: 29.10.14 | Page: 1 of 24 © Crown copyright 2014 Identification of Corynebacterium species Acknowledgments UK Standards for Microbiology Investigations (SMIs) are developed under the auspices of Public Health England (PHE) working in partnership with the National Health Service (NHS), Public Health Wales and with the professional organisations whose logos are displayed below and listed on the website https://www.gov.uk/uk- standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical- laboratories. SMIs are developed, reviewed and revised by various working groups which are overseen by a steering committee (see https://www.gov.uk/government/groups/standards-for-microbiology-investigations- steering-committee). The contributions of many individuals in clinical, specialist and reference laboratories who have provided information and comments during the development of this document are acknowledged. We are grateful to the Medical Editors for editing the medical content. For further information please contact us at: Standards Unit Microbiology Services Public Health England 61 Colindale Avenue London NW9 5EQ E-mail: [email protected] Website: https://www.gov.uk/uk-standards-for-microbiology-investigations-smi-quality- and-consistency-in-clinical-laboratories UK Standards for Microbiology Investigations are produced in association with: Logos correct at time of publishing. Bacteriology – Identification | ID 2 | Issue no: 4.1 | Issue date: 29.10.14 | Page: 2 of 24 UK Standards for Microbiology Investigations | Issued by the Standards Unit, Public Health England Identification of Corynebacterium species Contents ACKNOWLEDGMENTS ......................................................................................................... -
FROM DNA to BEER Date Lesson Plan: Acquired and Passive Immunity Class Period
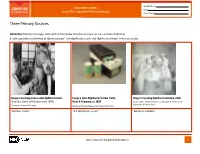
Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, ca. 1898 Courtesy The Historical Medical Library of The College of Physicians of Philadelphia Courtesy Library of Congress Courtesy National Museum of American History “diphtheria toxin”: “anti-diphtheritic serum”: “diphtheria antitoxin”: http://www.nlm.nih.gov/fromdnatobeer 1 Student Name FROM DNA TO BEER Date Lesson Plan: Acquired and Passive Immunity Class Period Three Primary Sources 2. Describe or draw how the three images may be related. http://www.nlm.nih.gov/fromdnatobeer 2 FROM DNA TO BEER Lesson Plan: Acquired and Passive Immunity Teacher’s Three Primary Sources Instruction: Examine the images and read their titles below. Based on what you can learn and infer from them: 1. Write your inferred definition of “diphtheria toxin,” “anti-diphtheritic serum,” and “diphtheria antitoxin” in the table below. Image 1. Injecting a horse with diphtheria toxin, Image 2. Anti-Diphtheritic Serum, Parke, Image 3. Injecting diphtheria antitoxin, 1895 New York City Health Department, 1940s Davis & Company, -

Biological Toxins Fact Sheet
Work with FACT SHEET Biological Toxins The University of Utah Institutional Biosafety Committee (IBC) reviews registrations for work with, possession of, use of, and transfer of acute biological toxins (mammalian LD50 <100 µg/kg body weight) or toxins that fall under the Federal Select Agent Guidelines, as well as the organisms, both natural and recombinant, which produce these toxins Toxins Requiring IBC Registration Laboratory Practices Guidelines for working with biological toxins can be found The following toxins require registration with the IBC. The list in Appendix I of the Biosafety in Microbiological and is not comprehensive. Any toxin with an LD50 greater than 100 µg/kg body weight, or on the select agent list requires Biomedical Laboratories registration. Principal investigators should confirm whether or (http://www.cdc.gov/biosafety/publications/bmbl5/i not the toxins they propose to work with require IBC ndex.htm). These are summarized below. registration by contacting the OEHS Biosafety Officer at [email protected] or 801-581-6590. Routine operations with dilute toxin solutions are Abrin conducted using Biosafety Level 2 (BSL2) practices and Aflatoxin these must be detailed in the IBC protocol and will be Bacillus anthracis edema factor verified during the inspection by OEHS staff prior to IBC Bacillus anthracis lethal toxin Botulinum neurotoxins approval. BSL2 Inspection checklists can be found here Brevetoxin (http://oehs.utah.edu/research-safety/biosafety/ Cholera toxin biosafety-laboratory-audits). All personnel working with Clostridium difficile toxin biological toxins or accessing a toxin laboratory must be Clostridium perfringens toxins Conotoxins trained in the theory and practice of the toxins to be used, Dendrotoxin (DTX) with special emphasis on the nature of the hazards Diacetoxyscirpenol (DAS) associated with laboratory operations and should be Diphtheria toxin familiar with the signs and symptoms of toxin exposure. -

The Influence of Social Conditions Upon Diphtheria, Measles, Tuberculosis and Whooping Cough in Early Childhood in London
VOLUME 42, No. 5 OCTOBER 1942 THE INFLUENCE OF SOCIAL CONDITIONS UPON DIPHTHERIA, MEASLES, TUBERCULOSIS AND WHOOPING COUGH IN EARLY CHILDHOOD IN LONDON BY G. PAYLING WRIGHT AND HELEN PAYLING WRIGHT, From the Department of Pathology-, Guy's Hospital Medical School (With 1 Figure in the Text) Before the war diphtheria, measles, tuberculosis and whooping cough were the most important of the better-defined causes of death amongst young children in the London area. The large numbers of deaths registered from these four diseases in the age group 0-4 years in the Metropolitan Boroughs alone between 1931 and 1938, together with the deaths recorded under bronchitis and pneumonia, are set out in Table 1. These records Table 1. Deaths from diphtheria, measles, tuberculosis (all forms), whooping cough, bron- chitis and pneumonia amongst children, 0-4 years, in the Metropolitan Boroughs from 1931 to 1938 Whooping Year Diphtheria Measles Tuberculosis cough Bronchitis Pneumonia 1931 148 109 184 301 195 1394 1932 169 760 207 337 164 1009 1933 163 88 150 313 101 833 1934 232 783 136 ' 277 167 1192 1935 125 17 108 161 119 726 1936 113 539 122 267 147 918 1937 107 21 100 237 122 827 1938 90 217 118 101 109 719 for diphtheria, measles, tuberculosis and whooping cough fail, however, to show all the deaths that should properly be ascribed to these specific diseases. For the most part, the figures represent the deaths occurring during their more acute stages, and necessarily omit some of the many instances in which these infections, after giving rise to chronic disabilities, terminate fatally from some less well-specified cause. -

Elizabeth Gyamfi
University of Ghana http://ugspace.ug.edu.gh GENOTYPING AND TREATMENT OF SECONDARY BACTERIAL INFECTIONS AMONG BURULI ULCER PATIENTS IN THE AMANSIE CENTRAL DISTRICT OF GHANA BY ELIZABETH GYAMFI (10442509) THIS THESIS IS SUBMITTED TO THE UNIVERSITY OF GHANA, LEGON IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF A MASTER OF PHILOSOPHY DEGREE IN MEDICAL BIOCHEMISTRY JULY, 2015 University of Ghana http://ugspace.ug.edu.gh DECLARATION I ELIZABETH GYAMFI, do hereby declare that with the exception of references to other people’s work, which have been duly acknowledged, this thesis is the outcome of my own research conducted at the Department of Medical Biochemistry, University of Ghana Medical School, College of Health Sciences and the Department of Cell, Molecular Biology and Biochemistry, University of Ghana, College of Basic and Applied Science under the supervision of Dr. Lydia Mosi and Dr. Bartholomew Dzudzor. Neither all nor parts of this project have been presented for another degree elsewhere. ……………………………………………. Date: ………………………. ELIZABETH GYAMFI (Student) ……………………………………………. Date: ………………………… DR. LYDIA MOSI (Supervisor) ………………………………………….. Date: ……………………….. DR. BATHOLOMEW DZUDZOR (Supervisor) i University of Ghana http://ugspace.ug.edu.gh ABSTRACT Background Buruli ulcer (BU) is a skin disease caused by Mycobacterium ulcerans. BU is the third most common mycobacterial disease after tuberculosis and leprosy, but in Ghana and Cote d’ Ivoire, it is the second. M. ulcerans produces mycolactone, an immunosuppressant macrolide toxin which makes the infection painless. However, some patients have complained of painful lesions and delay healing. Painful ulcers and delay healing experienced by some patients may be due to secondary bacterial infections. Main Objective: To identify secondary microbial infections of BU patients, their genetic diversity as well as determine the levels of antibiotics resistance of these microorganisms. -

Targeted Neuronal Cell Ablation in the Drosophila Embryo: Pathfinding by Follower Growth Cones in the Absence of Pioneers
Neuron, Vol. 14, 707-715, April, 1995, Copyright© 1995 by Cell Press Targeted Neuronal Cell Ablation in the Drosophila Embryo: Pathfinding by Follower Growth Cones in the Absence of Pioneers David M. Lin,* Vanessa J. Auld,*t reach their targets in the CNS (Wigglesworth, 1953). The and Corey S. Goodman ability of the initial axons to guide later growth cones has Howard Hughes Medical Institute led to the suggestion that pioneering growth cones might Division of Neurobiology be endowed with special pathfinding abilities. Do subse- Department of Molecular and Cell Biology quent growth cones possess the same pathfinding abili- Life Science Addition, Room 519 ties, or are they different from the pioneers? University of California, Berkeley Ablation experiments in a variety of organisms thus far Berkeley, California 94720 have provided a range of conflicting answers to this ques- tion. In the grasshopper embryo CNS, the G growth cone turns anteriorly along the P axons in the NP fascicle, a Summary pathway formed by the three descending P and the two ascending A axons. Although the A and P axons normally We developed a rapid method that uses diphtheria fasciculate and follow one another, either can pioneer the toxin, the flp recognition target sequences, and the complete pathway on its own. Furthermore, any one of GAL4-UAS activation system, to ablate specific neu- the P axons will suffice, since when any two of them are rons in the Drosophila embryo and to examine the con- ablated the remaining P pioneers the pathway. When the sequences in large numbers of embryos at many time three P axons are ablated, the G growth cone stalls, and points. -

(ACIP) General Best Guidance for Immunization
Appendix 1: Glossary Adverse event. An undesirable medical condition that occurs following vaccination which might be truly caused by a vaccine, or it might be pure coincidence. Adverse reaction. An undesirable medical condition that has been demonstrated to be caused by a vaccine. Evidence for the causal relation is usually obtained through randomized clinical trials, controlled epidemiologic studies, isolation of the vaccine strain from the pathogenic site, or recurrence of the condition with repeated vaccination (i.e., rechallenge); synonyms include side effect and adverse effect. Adjuvant. A vaccine component distinct from the antigen that enhances the immune response to the antigen. Antitoxin. A solution of antibodies against a toxin. Antitoxin can be derived from either human (e.g., tetanus immune globulin) or animal (usually equine) sources (e.g., diphtheria and botulism antitoxin). Antitoxins are used to confer passive immunity and for treatment. Hyperimmune globulin (specific). Special preparations obtained from blood plasma from donor pools preselected for a high antibody content against a specific antigen (e.g., hepatitis B immune globulin, varicella-zoster immune globulin, rabies immune globulin, tetanus immune globulin, vaccinia immune globulin, cytomegalovirus immune globulin, botulism immune globulin). Immune globulin. A sterile solution containing antibodies, which are usually obtained from human blood. It is obtained by cold ethanol fractionation of large pools of blood plasma and contains 15%-18% protein. Intended for intramuscular administration, immune globulin is primarily indicated for routine maintenance of immunity among certain immunodeficient persons and for passive protection against measles and hepatitis A. General Best Practice Guidelines for Immunization: Appendix 1: Glossary 189 Immunobiologic. Antigenic substances (e.g., vaccines and toxoids) or antibody- containing preparations (e.g., globulins and antitoxins) from human or animal donors. -

NGS-Based Phylogeny of Diphtheria-Related Pathogenicity Factors in Different Corynebacterium Spp
Dangel et al. BMC Microbiology (2019) 19:28 https://doi.org/10.1186/s12866-019-1402-1 RESEARCHARTICLE Open Access NGS-based phylogeny of diphtheria-related pathogenicity factors in different Corynebacterium spp. implies species- specific virulence transmission Alexandra Dangel1* , Anja Berger1,2*, Regina Konrad1 and Andreas Sing1,2 Abstract Background: Diphtheria toxin (DT) is produced by toxigenic strains of the human pathogen Corynebacterium diphtheriae as well as zoonotic C. ulcerans and C. pseudotuberculosis. Toxigenic strains may cause severe respiratory diphtheria, myocarditis, neurological damage or cutaneous diphtheria. The DT encoding tox gene is located in a mobile genomic region and tox variability between C. diphtheriae and C. ulcerans has been postulated based on sequences of a few isolates. In contrast, species-specific sequence analysis of the diphtheria toxin repressor gene (dtxR), occurring both in toxigenic and non-toxigenic Corynebacterium species, has not been done yet. We used whole genome sequencing data from 91 toxigenic and 46 non-toxigenic isolates of different pathogenic Corynebacterium species of animal or human origin to elucidate differences in extracted DT, DtxR and tox-surrounding genetic elements by a phylogenetic analysis in a large sample set. Results: Sequences of both DT and DtxR, extracted from whole genome sequencing data, could be classified in four distinct, nearly species-specific clades, corresponding to C. diphtheriae, C. pseudotuberculosis, C. ulcerans and atypical C. ulcerans from a non-toxigenic toxin gene-bearing wildlife cluster. Average amino acid similarities were above 99% for DT and DtxR within the four groups, but lower between them. For DT, subgroups below species level could be identified, correlating with different tox-comprising mobile genetic elements. -

Diphtheria Toxin
Duke University / Duke University Health System Policy on working with: Diphtheria Toxin I. Revision Page II. Background III. Risk Assessment IV. Policy Statement V. Emergency Information VI. Appendix I: EOHW Exposure/Injury Response Protocol VII. Appendix II: OESO SOP Template VIII. References This policy was first approved on December, 2018. Occupational and Environmental Safety Office (OESO) Employee Occupational Health and Wellness (EOHW) Page | 1 I. Revision Page Date Revision(s) / Comment(s) 12/06/2018 New policy document Page | 2 II. Background Diphtheria toxin (DT) is a biological toxin and is secreted by the bacterium Corynebacterium diphtheriae. DT is useful in biomedical research using mice because it can be used to selectively target and kill cells or organs without requiring surgery. Wild type mice do not have DT receptors, so they are relatively resistant to DT. The median lethal dose (LD50) for mice has been estimated at 1.6 mg/kg by subcutaneous injection compared to an estimated human LD50 of less than 100 ng/kg by IM injection. The gene for DT receptors can be inserted into a mouse genome so that the transgenic mice will express DT receptors only on specific cells. For example, a transgenic mouse engineered to express DT receptors only on hepatocytes can be injected with DT, which will only kill the hepatocytes, creating a nonsurgical mouse model without a functional liver. Diphtheria toxin doses used in transgenic mice range from 0.5 µg/kg to 50 µg/kg depending on the scientific goal (10 to 1000 ng for a 20-gram mouse).[Saito et al, 2001; Cha et al, 2003; Cerpa et al, 2014] A common dose is 100 ng per injection, sometimes administered in repeated doses to achieve a cumulative effect. -

Information Sheet Immunization and TB Requirements for Health Career Programs
Information Sheet Immunization and TB Requirements For Health Career Programs Why is immunization important for students in health career programs? Health care providers are at risk of exposure to, and possible transmission of, preventable diseases. Risk of communicable diseases in the workplace is due to health care providers contact with infected patients or infective material from patients. Maintenance of immunity is therefore an essential part of prevention and infection control. Vaccinating health care providers helps protect their health and prevent disease transmission between patients and providers and among providers and their family and friends outside the workplace. What routine immunizations and screenings are required? The vaccines required are Tetanus, Diphtheria, and Pertussis (Tdap), Measles (Rubeola), Mumps and Rubella (MMR), Varicella, and Influenza. Positive Rubella Titer is required in addition to MMR vaccination. Students must also be tested for TB on an annual basis. Tetanus, Diphtheria and Pertussis (required) Diphtheria is a serious communicable disease, causing death in 5-10 percent of cases with the highest rates among the very young and the elderly. Diphtheria disease is most common and most severe in non-immunized or partially immunized individuals. Protection from vaccine decreases over time unless periodic boosters are given. Tetanus is an acute and often fatal disease. While rare, cases have been reported that are associated with injection drug use, animal bites and wounds contaminated with dirt, feces or saliva. Pertussis (whooping cough) is very contagious and can cause serious illness―especially in infants too young to be fully vaccinated. Pertussis vaccines are recommended for children, teens, and adults, including pregnant women Immunization against tetanus, diphtheria, and pertussis is recommended for all adults in the USA and required for students in health career programs at HACC. -

Cold Sensing by Nav1.8-Positive and Nav1.8-Negative Sensory Neurons
Cold sensing by NaV1.8-positive and NaV1.8-negative sensory neurons A. P. Luiza, D. I. MacDonalda, S. Santana-Varelaa, Q. Milleta, S. Sikandara, J. N. Wooda,1, and E. C. Emerya,1 aMolecular Nociception Group, Wolfson Institute for Biomedical Research, University College London, London WC1E 6BT, United Kingdom Edited by Peter McNaughton, King’s College London, London, United Kingdom, and accepted by Editorial Board Member David E. Clapham January 8, 2019 (received for review August 23, 2018) The ability to detect environmental cold serves as an important define the distribution and identity of cold-sensitive DRG neurons, survival tool. The sodium channels NaV1.8 and NaV1.9, as well as in live mice, using in vivo imaging. the TRP channel Trpm8, have been shown to contribute to cold sensation in mice. Surprisingly, transcriptional profiling shows that Results NaV1.8/NaV1.9 and Trpm8 are expressed in nonoverlapping neuro- Distribution of DRG Sensory Neurons Responsive to Noxious Cold, in nal populations. Here we have used in vivo GCaMP3 imaging to Vivo. To identify cold-sensitive neurons in vivo, we used a pre- identify cold-sensing populations of sensory neurons in live mice. viously developed in vivo imaging technique to study the responses We find that ∼80% of neurons responsive to cold down to 1 °C do of individual DRG neurons in situ (8). Mice coexpressing Pirt- not express NaV1.8, and that the genetic deletion of NaV1.8 does GCaMP3 (which enables pan-DRG GCaMP3 expression), not affect the relative number, distribution, or maximal response NaV1.8 Cre, and a Cre-dependent reporter (tdTomato) were of cold-sensitive neurons. -

(Buruli Ulcer) in Rural Hospital, Southern Benin, 1997–2001 Martine Debacker,* Julia Aguiar,† Christian Steunou,† Claude Zinsou,*† Wayne M
Mycobacterium ulcerans Disease (Buruli Ulcer) in Rural Hospital, Southern Benin, 1997–2001 Martine Debacker,* Julia Aguiar,† Christian Steunou,† Claude Zinsou,*† Wayne M. Meyers,‡ Augustin Guédénon,§ Janet T. Scott,* Michèle Dramaix,¶ and Françoise Portaels* Data from 1,700 patients living in southern Benin were Even though large numbers of patients have been collected at the Centre Sanitaire et Nutritionnel Gbemoten, reported, the epidemiology of BU remains obscure, even in Zagnanado, Benin, from 1997 through 2001. In the Zou disease-endemic countries. In 1997, a first report was pub- region in 1999, Buruli ulcer (BU) had a higher detection rate lished on 867 BU patients from the Republic of Benin (21.5/100,000) than leprosy (13.4/100,000) and tuberculo- (West Africa) for 1989–1996 (4). Our study covers the sis (20.0/100,000). More than 13% of the patients had osteomyelitis. Delay in seeking treatment declined from 4 ensuing 5 years (1997 to 2001), during which a collabora- months in 1989 to 1 month in 2001, and median hospital- tive project was initiated to improve detection and control ization time decreased from 9 months in 1989 to 1 month of BU. This study describes BU in Benin and presents in 2001. This reduction is attributed, in part, to implement- demographic trends and epidemiologic data from the four ing an international cooperation program, creating a nation- southern regions of Benin (Zou, Oueme, Mono, and al BU program, and making advances in patient care. Atlantique), as seen in a rural hospital in the Zou Region. uruli ulcer (BU), caused by Mycobacterium ulcerans, Patients and Methods Bis the third most common mycobacterial disease in Our observations are based on 1,700 consecutive humans after tuberculosis and leprosy (1).