Investigator's Brochure: Vaccinia Immune Globulin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

(ACIP) General Best Guidance for Immunization
Appendix 1: Glossary Adverse event. An undesirable medical condition that occurs following vaccination which might be truly caused by a vaccine, or it might be pure coincidence. Adverse reaction. An undesirable medical condition that has been demonstrated to be caused by a vaccine. Evidence for the causal relation is usually obtained through randomized clinical trials, controlled epidemiologic studies, isolation of the vaccine strain from the pathogenic site, or recurrence of the condition with repeated vaccination (i.e., rechallenge); synonyms include side effect and adverse effect. Adjuvant. A vaccine component distinct from the antigen that enhances the immune response to the antigen. Antitoxin. A solution of antibodies against a toxin. Antitoxin can be derived from either human (e.g., tetanus immune globulin) or animal (usually equine) sources (e.g., diphtheria and botulism antitoxin). Antitoxins are used to confer passive immunity and for treatment. Hyperimmune globulin (specific). Special preparations obtained from blood plasma from donor pools preselected for a high antibody content against a specific antigen (e.g., hepatitis B immune globulin, varicella-zoster immune globulin, rabies immune globulin, tetanus immune globulin, vaccinia immune globulin, cytomegalovirus immune globulin, botulism immune globulin). Immune globulin. A sterile solution containing antibodies, which are usually obtained from human blood. It is obtained by cold ethanol fractionation of large pools of blood plasma and contains 15%-18% protein. Intended for intramuscular administration, immune globulin is primarily indicated for routine maintenance of immunity among certain immunodeficient persons and for passive protection against measles and hepatitis A. General Best Practice Guidelines for Immunization: Appendix 1: Glossary 189 Immunobiologic. Antigenic substances (e.g., vaccines and toxoids) or antibody- containing preparations (e.g., globulins and antitoxins) from human or animal donors. -

The Study of Thimerosal and Autism
The Study of Thimerosal and Autism Documentation and Codebook for the Child Vaccination Histories File: Cambridge, MA Lexington, MA Hadley, MA Bethesda, MD September 3, 2010 Chicago, IL Prepared for Frank DeStefano National Immunization Program Centers for Disease Control and Prevention Atlanta, GA 30329 Prepared by Cristofer Price Yeqin He Abt Associates Inc. Suite 800 North 4550 Montgomery Avenue Bethesda, MD 20814-3343 This documentation was prepared by Cristofer Price and Yeqin He of Abt Associates Inc. for the Immunization Safety Office (ISO) of the Centers for Disease Control and Prevention (CDC) Atlanta, GA 30333. Questions about the documentation, substantive questions, or technical issues regarding the data file should be directed to the CDC ISO, MS D26, 1600 Clifton Road, Atlanta, Georgia 30333 (404-639-8256). Suggested Citation for Study of Thimerosal and Autism Public Use Data Set: Price, C.S., He, Y. (2010) The Study of Thimerosal and Autism: Data Set Documentation. Bethesda MD: Abt Associates, Inc; Prepared for the National Immunization Program Centers for Disease Control and Prevention Atlanta, GA Abt Associates Inc. 1 Documentation and Codebook for the Child Vaccination Histories File 1. Introduction to the Child Vaccination Histories File............................................... 2 2. File Formats and Variable Descriptions................................................................... 4 1. Introduction to the Child Vaccination Histories File The Child Vaccination Histories File contains the data that were used to construct the measures of early childhood (postnatal) exposure to ethylmercury from thimerosal- containing vaccines and immune globulin preparations received by the study children during the age range spanning birth to 20 months. The Child Vaccination Histories File is provided with the public use data in order to make the calculation of exposure amounts transparent, and so that analysts have the potential to calculate alternative measures of exposure1. -

Mass Vaccination: When and Why
CTMI (2006) 304:1–16 Mass Vaccination: When and Why D. L. Heymann (u)·R.B.Aylward World Health Organization, 1211 Geneva 27, Switzerland [email protected] 1 The Concept of Mass Vaccination ............................. 2 2 Mass vaccination in the Twentieth Century—Smallpox Eradication .... 3 3 Routine Immunization and Mass Vaccination Today— A Necessary Alliance ..................................... 4 4 Preventing Emerging Outbreaks ............................. 5 4.1 Meningitis............................................. 6 4.2 Influenza.............................................. 7 4.3 YellowFever............................................ 8 4.4 DisplacedPersons....................................... 8 4.5 ThreatofDeliberatelyCausedOutbreaks....................... 9 5 Mass Vaccination to Accelerate Disease Control .................. 10 5.1 NewVaccineIntroduction.................................. 11 5.2 Eradication............................................ 12 5.3 MortalityReduction...................................... 13 5.3.1 Measles............................................... 13 5.3.2 MaternalandNeonatalTetanus.............................. 13 6 Mass Vaccination in the Twenty-First Century .................... 13 References .................................................. 14 Abstract With increased demand for smallpox vaccination during the nineteenth cen- tury, vaccination days—early mass vaccination campaigns—were conducted over time-limited periods to rapidly and efficiently protect maximum numbers of suscepti- ble persons. -

Impact of Vaccine Type on HIV-1 Vaccine Elicited Antibody Durability
www.nature.com/scientificreports OPEN Impact of vaccine type on HIV‑1 vaccine elicited antibody durability and B cell gene signature Rohith Palli1,2,15, Kelly E. Seaton3,15, Michael S. Piepenbrink4, John Hural5, Paul A. Goepfert4, Fatima Laher6, Susan P. Buchbinder7, Gavin Churchyard8, Glenda E. Gray6,9, Harriet L. Robinson10, Yunda Huang5, Holly Janes5,11, James J. Kobie4, Michael C. Keefer12, Georgia D. Tomaras3 & Juilee Thakar13,14* Efcacious HIV‑1 vaccination requires elicitation of long‑lived antibody responses. However, our understanding of how diferent vaccine types elicit durable antibody responses is lacking. To assess the impact of vaccine type on antibody responses, we measured IgG isotypes against four consensus HIV antigens from 2 weeks to 10 years post HIV‑1 vaccination and used mixed efects models to estimate half‑life of responses in four human clinical trials. Compared to protein‑boosted regimens, half‑lives of gp120‑specifc antibodies were longer but peak magnitudes were lower in Modifed Vaccinia Ankara (MVA)‑boosted regimens. Furthermore, gp120‑specifc B cell transcriptomics from MVA‑boosted and protein‑boosted vaccines revealed a distinct signature at a peak (2 weeks after last vaccination) including CD19, CD40, and FCRL2‑5 activation along with increased B cell receptor signaling. Additional analysis revealed contributions of RIG‑I‑like receptor pathway and genes such as SMAD5 and IL‑32 to antibody durability. Thus, this study provides novel insights into vaccine induced antibody durability and B‑cell receptor signaling. While vaccine-elicited antibody durability has been achieved for licensed vaccines such as yellow fever, measles, smallpox, and Hepatitis B, elicitation of long-lived, functional antibody responses with candidate HIV-1 vac- cine regimens remains elusive1,2. -

Chapter 7. Developments in Vaccination and Control Between
CHAPTER 7 DEVELOPMENTS IN VACCINATION AND CONTROL BETWEEN 1900 AND 1966 Contents Page Introduction 277 Vaccine production and quality control before 1967 278 Production of vaccine lymph 279 Preparation of liquid vaccine 282 Preparation of dried vaccine 283 Quality control 289 Vaccination techniques before 1967 291 Vaccination site 291 Methods of vaccination 292 Age for primary vaccination 293 Interpretation of the results of vaccination 294 Complications of vaccination 296 Types of complication 299 Frequency of complications 302 Contraindications to vaccination 307 Prevention and treatment of complications 308 Reconsideration of vaccination policies in non-endemic countries 309 Complications : the overall picture 310 Programmes for vaccination and revaccination 311 Vaccination and revaccination of the general public 311 Simultaneous vaccination with several antigens 311 Vaccination of international travellers 312 INTRODUCTION The latter half of the 19th century saw the emergence of microbiology and immunology By the year 1900 vaccination was in as scientific disciplines . Because of their widespread use throughout the industrialized familiarity with vaccination, many of the countries as well as in some cities in what were pioneers in these new sciences used vaccinia then the colonies of various European virus for their studies (see Chapter 2) . In powers . Although variolation was no longer consequence, the empirical practices of Jenner practised in Europe and North America, it and his early followers were placed on a more was still widely employed in many parts of scientific basis . Vaccine production was no Africa and Asia . Smallpox persisted as an longer the province of the local physician, endemic disease in virtually every country of who had maintained the virus by arm-to-arm the world (see Chapter 8, Fig. -

INTRODUCTION We Invite You to Take Part in a Research Study at The
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY MEDICAL RECORD • Adult Patient or • Parent, for Minor Patient INSTITUTE: National Cancer Institute STUDY NUMBER: 16-C-0035 PRINCIPAL INVESTIGATOR: Ravi Madan, M.D. STUDY TITLE: Prostvac in Patients with Biochemical Recurrent Prostate Cancer Continuing Review Approved by the IRB on 09/23/20 Amendment Approved by the IRB on 06/12/18 (H) Date posted to web: 9/26/20 Standard INTRODUCTION We invite you to take part in a research study at the National Institutes of Health (NIH). First, we want you to know that: Taking part in NIH research is entirely voluntary. You may choose not to take part, or you may withdraw from the study at any time. In either case, you will not lose any benefits to which you are otherwise entitled. However, to receive care at the NIH, you must be taking part in a study or be under evaluation for study participation. You may receive no benefit from taking part. The research may give us knowledge that may help people in the future. Second, some people have personal, religious or ethical beliefs that may limit the kinds of medical or research treatments they would want to receive (such as blood transfusions). If you have such beliefs, please discuss them with your NIH doctors or research team before you agree to the study. Now we will describe this research study. Before you decide to take part, please take as much time as you need to ask any questions and discuss this study with anyone at NIH, or with family, friends or your personal physician or other health professional. -

Therapeutic Vaccines and Antibodies for Treatment of Orthopoxvirus Infections
Viruses 2010, 2, 2381-2403; doi:10.3390/v2102381 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Therapeutic Vaccines and Antibodies for Treatment of Orthopoxvirus Infections Yuhong Xiao 1 and Stuart N. Isaacs 1,2,* 1 Division of Infectious Diseases, University of Pennsylvania, School of Medicine, Philadelphia, PA 19104, USA; E-Mail: [email protected] 2 Infectious Diseases Section, Philadelphia Veterans Affairs Medical Center, Philadelphia, PA 19104, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-215-662-2150; Fax: +1-215-349-5111. Received: 19 September 2010; in revised form: 9 October 2010 / Accepted: 13 October 2010 / Published: 20 October 2010 Abstract: Despite the eradication of smallpox several decades ago, variola and monkeypox viruses still have the potential to become significant threats to public health. The current licensed live vaccinia virus-based smallpox vaccine is extremely effective as a prophylactic vaccine to prevent orthopoxvirus infections, but because of safety issues, it is no longer given as a routine vaccine to the general population. In the event of serious human orthopoxvirus infections, it is important to have treatments available for individual patients as well as their close contacts. The smallpox vaccine and vaccinia immune globulin (VIG) were used in the past as therapeutics for patients exposed to smallpox. VIG was also used in patients who were at high risk of developing complications from smallpox vaccination. Thus post-exposure vaccination and VIG treatments may again become important therapeutic modalities. This paper summarizes some of the historic use of the smallpox vaccine and immunoglobulins in the post-exposure setting in humans and reviews in detail the newer animal studies that address the use of therapeutic vaccines and immunoglobulins in orthopoxvirus infections as well as the development of new therapeutic monoclonal antibodies. -
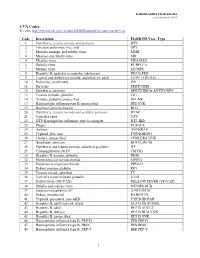
VALID VALUES Last Updated 3/4/20
FLORIDA SHOTS VALID VALUES Last Updated 3/4/20 CVX Codes See also: http://www2a.cdc.gov/vaccines/IIS/IISStandards/vaccines.asp?rpt=cvx Code Description FLSHOTS Vacc. Type 1 Diphtheria, tetanus toxoids and pertussis DTP 2 Trivalent poliovirus, live, oral OPV 3 Measles, mumps, and rubella virus MMR 4 Measles and rubella virus MR 5 Measles virus MEASLES 6 Rubella virus RUBELLA 7 Mumps virus MUMPS 8 Hepatitis B, pediatric or pediatric/adolescent HEP B PED 9 Tetanus and diphtheria toxoids, adsorbed, pf, adult TD PF (TDVAX) 10 Poliovirus, inactivated IPV 11 Pertussis PERTUSSIS 12 Diphtheria antitoxin DIPHTHERIA ANTITOXIN 13 Tetanus immune globulin TIG 14 Immune globulin, unspecified IG UNK 17 Haemophilus influenza type B, unspecified HIB UNK 19 Bacillus Calmette-Guerin BCG 20 Diphtheria, tetanus toxoids and acellular pertussis DTAP 21 Varicella virus VZV 22 DTP-Haemophilus influenzae type b conjugate DTP-HIB 23 Plague PLAGUE 24 Anthrax ANTHRAX 25 Typhoid, live, oral TYPHOID PO 26 Cholera, unspecified CHOLERA UNK 27 Botulinum antitoxin BOTULINUM 28 Diphtheria and tetanus toxoids, adsorbed, pediatric DT 29 Cytomagalovirus IG IV CMVIG 30 Hepatitis B immune globulin HBIG 32 Meningococcal polysaccharide MPSV4 33 Pneumoccocal polysaccharide PPSV23 34 Rabies immune globulin RIG 35 Tetanus toxoid, adsorbed TT 36 Varicella zoster immune globulin VZIG 37 Yellow fever (YF-VAX) YELLOW FEVER (YF-VAX) 38 Rubella and mumps virus MUMPS-RUB 39 Japanese encephalitis, SC JENCEPH SC 40 Rabies, intradermal RABIES ID 41 Typhoid, parenteral, non-AKD TYPHOID PAR 42 Hepatitis B, adol/high risk infant HEP B HIGH RISK 43 Hepatitis B, adult HEP B ADULT 44 Hepatitis B, dialysis HEP B DIALYSIS 45 Hepatitis B, unspecified HEP B UNK 46 Haemophilus influenza type B, PRP-D HIB PRP-D 47 Haemophilus influenza type B, HbOC HIB HBOC 48 Haemophilus influenza type B, PRP-T HIB PRP-T 1 FLORIDA SHOTS VALID VALUES Last Updated 3/4/20 Code Description FLSHOTS Vacc. -

Disease Eradication
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this site. Copyright 2007, The Johns Hopkins University and Kenrad Nelson. All rights reserved. Use of these materials permitted only in accordance with license rights granted. Materials provided “AS IS”; no representations or warranties provided. User assumes all responsibility for use, and all liability related thereto, and must independently review all materials for accuracy and efficacy. May contain materials owned by others. User is responsible for obtaining permissions for use from third parties as needed. Disease Eradication Kenrad Nelson, MD Johns Hopkins University Objectives Review issues related to the eradication of an infectious disease − Smallpox Polio − 3 Section A Smallpox Disease Eradication: Definitions Cockburn (1961) − …the complete extinction of the pathogen that causes infectious disease… so long as a single member of the species survives, the eradication has not been accomplished Andrews and Langmuir (1963) − The purposeful reduction of specific disease prevalence to the point of a continued absence of transmission within a specific area X Note the elements of geography, time, and implied surveillance X This is probably the “best” simple definition WHO (1980) for smallpox − …the eradication of clinical infection by variola virus… since there is no carrier state, no subclinical infection, and no animal reservoir… the absence of clinical disease implies the absence of disease transmission 5 Advantages of an Eradication Strategy Advantages of a strategy of eradication of an infectious disease 1. Once disease is truly eradicated, further control efforts can be abandoned, with savings of cost, adverse vaccine reactions, etc. -

Vaccines Could Halt Third World Health Crisis Vaccines by Anita Manning 2 Human Beings Are Born with the Bare Minimum of Defenses Against Infectious Diseases
THE NATION’S NEWSPAPER PTK2003-04 Collegiate Case Study www.usatodaycollege.com Vaccines could halt Third World health crisis Vaccines By Anita Manning 2 Human beings are born with the bare minimum of defenses against infectious diseases. It is the job of a healthy immune system to strengthen itself over Vaccine use spreading time by recognizing and adapting to new threats. When a new, potentially harmful microbe enters the body, the immune system provides defense By Gary Mihoces 2 against it in the form of antibodies. More importantly, the body will remember the attacker and remember how to kill it. This secondary response provides immunity from future infection. Vaccines increase our protection by stimulating the immune system to generate antibodies against specific antigens. Large-scale vaccination of the population, called herd immunity, also provides an important defense against infectious disease. Herd immunity limits outbreaks because there are not enough susceptible people to support an epidemic. Well before the development of vaccines, humans knew that prior infection to a disease could protect a person from getting that disease again. Recorded history provides many examples of vaccination techniques being used without a full understanding of the mechanisms that allow them to work. As early as the 10th century, Chinese physicians had children inhale dried and powdered smallpox scabs. In 18th Century England, a similar procedure was used when Photo by Tim Dillon, USA TODAY small pox scabs were scratched into children's skin. Unfortunately, in some cases the procedure did backfire and kill the recipient. However, the 1% Cancer vaccine shows mortality rate was a vast improvement over the 50% mortality of smallpox. -

Vaccines for the Third World Barry R
COMMENTARY Vaccines for the Third World Barry R. Bloom New vaccines, developed through genetic engineering, can make immunization an even more effective weapon for tackling disease in developing countries. So what is preventing progress? Where there is no vision, the people perish available, as well as inherent limitations in Any new or improved vaccine to be ... Proverbs 29:18 each of the currently used vaccines. Only considered for inclusion in the EPI two EPI vaccines- BCG, used to immu should, ideally, have the following attri THE Third World is the place where three nize against tuberculosis, and oral polio butes: (1) it must be inexpensive; (2) it quarters of the people of this planet live, vaccine-can be given at birth or any time must be safe; (3) it must be extremely where 86 per cent of all births and 96 per thereafter, and BCG and measles are the effective, inducing protection in 90-100 2 cent of child and infant deaths occur'· • At only vaccines that require a single immu per cent of recipients; (4) it should both a national and a human level the nizing dose. DPT and polio must be given engender lifetime immunity; (5) it should diverse problems of the developing be heat-stable and not need to be kept countries are of staggering proportions. ~ cold at all stages (a cold chain), which is Most have heavy foreign debt and, as ~ both expensive and subject to catastrophe; a consequence, these countries now (6) it should require only one shot or be transfer to the developed countries more compatible with the schedule for other hard currency than they receive ($31.1 vaccines; (7) it should be simple to give, billion in 1987)'.