The Study of Thimerosal and Autism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Varicella (Chickenpox): Questions and Answers Q&A Information About the Disease and Vaccines
Varicella (Chickenpox): Questions and Answers Q&A information about the disease and vaccines What causes chickenpox? more common in infants, adults, and people with Chickenpox is caused by a virus, the varicella-zoster weakened immune systems. virus. How do I know if my child has chickenpox? How does chickenpox spread? Usually chickenpox can be diagnosed by disease his- Chickenpox spreads from person to person by direct tory and appearance alone. Adults who need to contact or through the air by coughing or sneezing. know if they’ve had chickenpox in the past can have It is highly contagious. It can also be spread through this determined by a laboratory test. Chickenpox is direct contact with the fluid from a blister of a per- much less common now than it was before a vaccine son infected with chickenpox, or from direct contact became available, so parents, doctors, and nurses with a sore from a person with shingles. are less familiar with it. It may be necessary to perform laboratory testing for children to confirm chickenpox. How long does it take to show signs of chickenpox after being exposed? How long is a person with chickenpox contagious? It takes from 10 to 21 days to develop symptoms after Patients with chickenpox are contagious for 1–2 days being exposed to a person infected with chickenpox. before the rash appears and continue to be conta- The usual time period is 14–16 days. gious through the first 4–5 days or until all the blisters are crusted over. What are the symptoms of chickenpox? Is there a treatment for chickenpox? The most common symptoms of chickenpox are rash, fever, coughing, fussiness, headache, and loss of appe- Most cases of chickenpox in otherwise healthy children tite. -

(ACIP) General Best Guidance for Immunization
Appendix 1: Glossary Adverse event. An undesirable medical condition that occurs following vaccination which might be truly caused by a vaccine, or it might be pure coincidence. Adverse reaction. An undesirable medical condition that has been demonstrated to be caused by a vaccine. Evidence for the causal relation is usually obtained through randomized clinical trials, controlled epidemiologic studies, isolation of the vaccine strain from the pathogenic site, or recurrence of the condition with repeated vaccination (i.e., rechallenge); synonyms include side effect and adverse effect. Adjuvant. A vaccine component distinct from the antigen that enhances the immune response to the antigen. Antitoxin. A solution of antibodies against a toxin. Antitoxin can be derived from either human (e.g., tetanus immune globulin) or animal (usually equine) sources (e.g., diphtheria and botulism antitoxin). Antitoxins are used to confer passive immunity and for treatment. Hyperimmune globulin (specific). Special preparations obtained from blood plasma from donor pools preselected for a high antibody content against a specific antigen (e.g., hepatitis B immune globulin, varicella-zoster immune globulin, rabies immune globulin, tetanus immune globulin, vaccinia immune globulin, cytomegalovirus immune globulin, botulism immune globulin). Immune globulin. A sterile solution containing antibodies, which are usually obtained from human blood. It is obtained by cold ethanol fractionation of large pools of blood plasma and contains 15%-18% protein. Intended for intramuscular administration, immune globulin is primarily indicated for routine maintenance of immunity among certain immunodeficient persons and for passive protection against measles and hepatitis A. General Best Practice Guidelines for Immunization: Appendix 1: Glossary 189 Immunobiologic. Antigenic substances (e.g., vaccines and toxoids) or antibody- containing preparations (e.g., globulins and antitoxins) from human or animal donors. -

Gsk Vaccines in 2010
GSK VACCINES IN 2010 Thomas Breuer, MD, MSc Senior Vice President Head of Global Vaccines Development GSK Biologicals Vaccines business characteristics Few global players and high barriers to entry – Complex manufacturing – Large scale investment Long product life cycles – Complex intellectual property High probability of R&D success – 70% post-POC New technology/novel products Better pricing for newer vaccines – HPV vaccines (Cervarix, Gardasil) – Pneumococcal vaccines (Synflorix, Prevnar-13) Operating margin comparable to pharmaceutical products Heightened awareness New markets 2 Research & development timelines Identify Produce Pre-Clinical Proof of Registration/ Phase I Phase II Phase III File Antigens Antigens Testing Concept Post Marketing Research (inc. Immunology) Pre-Clinical Development (inc. Formulation Science) Clinical Development (inc. Post Marketing Surveillance) Transfer Process to Manufacturing Build Facility x x Up to $10-20M Up to $50-100M $500M - $1B x x x 1-10 yrs 2-3 yrs 2-4 yrs > 1 yr GSK vaccines business 2009 sales £3.7 billion (+30%) +19% CAGR excl. H1N1 Vaccines represent 13% since 2005 of total GSK sales Sale s (£m) 4000 3500 Recent approvals: 3000 US: Cervarix 2500 EU: Synflorix 2000 Pandemic: Pandemrix; Arepanrix 1500 1000 500 0 2005 2006 2007 2008 2009 Increased Emerging Market presence Growth rate is CER 5 GSK vaccines: fastest growing part of GSK in 2009 2009 Sales Share Growth (CER) Respiratory £ 6,977m 25% +5% Consumer £ 4,654m 16% +7% Anti-virals £ 4,150m 15% +12% Vaccines £ 3,706m 13% +30% CV & Urogenital -
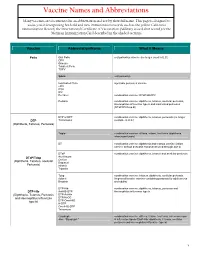
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Investigator's Brochure: Vaccinia Immune Globulin
Use of Vaccinia Immune Globulin (VIG) For Treatment of vaccinia vaccine complications and for prophylaxis of unvaccinated individuals exposed to Orthopoxviruses responsive to VIG Investigational New Drug (IND) Proposal TABLE OF CONTENTS: 1. Introduction............................................................Page 2 2. Effects in Humans..................................................Pages 3 - 4 3. Nonclinical Studies.................................................Pages 5- 6 4. Rationale and Objectives.........................................Page 7 5. Study Design... .......................................................Page 8 6. Additional Information - Selected References..........Pages 9 - 15 Page -1- INVESTIGATOR’S BROCHURE INTRODUCTION This Investigational New Drug Application is being filed in order to make existing stocks of Vaccinia Immune Globulin available for treatment of complications of vaccinia vaccination or exposure to vaccinia or related pox viruses. Vaccinia Immune Globulin (VIG) (Human) is an isotonic sterile solution of the immunoglobulin fraction of plasma from persons vaccinated with vaccinia vaccine. The current lot, 0448A101A, was released as a licensed product by Baxter Healthcare Corporation in 1995. In 1998 a slight discoloration was noted in this product and the FDA placed a hold on its release. Consequently, VIG can no longer be entered into interstate commerce under terms of the approved license and must therefore revert to IND (investigational new drug) status. CDC follows the June 22, 2001 Recommendations of the Immunization Practices Advisory Committee (ACIP) for Vaccinia (Smallpox) Vaccine. Since 1983 CDC has provided vaccinia vaccine for laboratory workers directly involved with smallpox or closely related orthopox viruses (e.g., monkeypox and vaccinia). Due to clinical trials involving recombinant vaccinia virus vaccines, health-care workers (e.g., physicians and nurses) may now be exposed to vaccinia and recombinant vaccinia viruses and may be considered for vaccinia vaccination. -

Vaccine-Associated Paralytic Poliomyelitis and BCG-Osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013
Morbidity and Mortality Weekly Report Weekly / Vol. 63 / No. 33 August 22, 2014 Vaccine-Associated Paralytic Poliomyelitis and BCG-osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013 Robert Trimble, MD1, Jane Atkins, MD2, Troy C. Quigg, DO3, Cara C. Burns, PhD4, Gregory S. Wallace, MD4, Mary Thomas, MBBS5, Anil T. Mangla, PhD5, Anthony J. Infante, MD, PhD1 (Author affiliations at end of text) Poliovirus transmission has been eliminated in most of developed VAPP (2). A case of immunodeficiency-associated the world through the use of inactivated poliovirus vaccine vaccine-derived poliovirus (iVDPV) infection, without paraly- (IPV) and live, attenuated oral poliovirus vaccine (OPV). In sis, was diagnosed in an unvaccinated child with SCIDS in the United States, use of OPV was discontinued by the year 2005, but the source of the virus could not be definitively 2000 because of the potential for vaccine-associated paralytic identified (3). A woman in Minnesota aged 44 years with polio (VAPP); an average of eight cases were reported each long-standing common variable immunodeficiency died after year in the United States during 1980–2000 (1). Polio eradi- developing VAPP in 2009 (4). She was probably infected when cation efforts in other parts of the world continue to rely on her child received OPV approximately 12 years earlier. Case OPV to take advantage of transmission of poliovirus vaccine reports and cohort studies from several countries other than strains to unvaccinated persons in the population, lower cost, the United States demonstrate the continued occurrence of and ease of administration. In 2013, an infant aged 7 months iVDPVs and the need for ongoing surveillance (5). -

Global Advisory Committee on Vaccine Safety (GACVS)
Global Advisory Committee on Vaccine Safety (GACVS) Report on GACVS meeting June 2013 1 | GACVS June 2013 report Topics Discussed ! Pentavalent vaccine in 4 Asian Countries ! Zoster vaccine safety and varicella vaccine safety in immunocompromised populations ! Immunization during pregnancy ! Yellow fever vaccine safety during mass immunization campaigns in sub-Saharan Africa ! Safety profile: Japanese encephalitis vaccines ! Update: human papillomavirus vaccines ! Update: pandemic influenza vaccine (Pandemrix®) and narcolepsy 2 | GACVS June 2013 report Progressive pentavalent vaccine introduction in 4 Asian Countries ! Sri Lanka (Crucell, Jan 2008): – Within 3 months, 4 deaths and 24 suspected HHE: precautionary suspension of initial lot. – 1 death following immunization April 2009: vaccine suspended, DTwP and Hep B resumed. ! Bhutan (Panacea, Sep 2009) – 5 cases of encephalopathy and/or meningoencephalitis lead to suspended vaccination 23 Oct 2009. (Subsequently, 4 serious AEFI were identified and investigated). ! India (Serum Institute of India, Tamil Nadu and Kerala, Dec 2011; Goa, Pondicherry, Karnataka, Haryana, Jammu and Kashmir, Gujarat and Delhi from Q3 2012 – Q1 2013) – To date, 83 AEFI cases reported, some associated with mortality. ! Vietnam (Crucell, Jun 2010 - May 2013) – 43 serious AEFI investigated, including 27 with fatal outcome. – Following 9 deaths following vaccination reported Dec 2012 - March 2013: vaccine suspended. 3 | GACVS June 2013 report GACVS analysis of common features among countries experiencing significant vaccine safety concerns ! Vaccination programmes are well established and achieves high coverage ! Vaccine introduction was accompanied by thorough training of health-care staff on benefits and risks of vaccine ! Sri Lanka and Bhutan: discontinuation and resumption of pentavalent vaccine did not significantly modify pattern of serious AEFI with previously utilized vaccines ! Limitations in all 4 countries: – Incomplete clinical information complicated causality assessment. -

Mass Vaccination: When and Why
CTMI (2006) 304:1–16 Mass Vaccination: When and Why D. L. Heymann (u)·R.B.Aylward World Health Organization, 1211 Geneva 27, Switzerland [email protected] 1 The Concept of Mass Vaccination ............................. 2 2 Mass vaccination in the Twentieth Century—Smallpox Eradication .... 3 3 Routine Immunization and Mass Vaccination Today— A Necessary Alliance ..................................... 4 4 Preventing Emerging Outbreaks ............................. 5 4.1 Meningitis............................................. 6 4.2 Influenza.............................................. 7 4.3 YellowFever............................................ 8 4.4 DisplacedPersons....................................... 8 4.5 ThreatofDeliberatelyCausedOutbreaks....................... 9 5 Mass Vaccination to Accelerate Disease Control .................. 10 5.1 NewVaccineIntroduction.................................. 11 5.2 Eradication............................................ 12 5.3 MortalityReduction...................................... 13 5.3.1 Measles............................................... 13 5.3.2 MaternalandNeonatalTetanus.............................. 13 6 Mass Vaccination in the Twenty-First Century .................... 13 References .................................................. 14 Abstract With increased demand for smallpox vaccination during the nineteenth cen- tury, vaccination days—early mass vaccination campaigns—were conducted over time-limited periods to rapidly and efficiently protect maximum numbers of suscepti- ble persons. -

Gardasil, Zostavax Shots: Questions and Answers
24 Infectious Diseases FAMILY P RACTICE N EWS • November 1, 2007 Gardasil, Zostavax Shots: Questions and Answers BY BETSY BATES tion in May 2006 for adults aged 60 years Gardasil companies probably will not cover the cost Los Angeles Bureau and older. Ǡ “I’m 30, and I’m in the dating scene. of the vaccine in children or young women A month later, Gardasil was approved for Should I get the Gardasil vaccine even outside of the FDA-specified age groups. M ONTEREY, CALIF. — Although girls and young women aged 9-26 years to though it’s only approved for ages 9-26?” Ǡ “What about vaccinating boys and most physicians are familiar with the ba- prevent cervical cancer, precancerous gen- The vaccine was studied in younger young men?” sic facts concerning the newly introduced ital lesions, and genital warts caused by women, but “there’s no reason in the Men obviously play an important role in Gardasil and Zostavax vaccines, patients HPV types 6, 11, 16, and 18. world in terms of safety and efficacy” that the cycle of spread of oncogenic papilloma still have many questions about their use. Dr. Stephen K. Tyring provided some older women who are sexually active viruses, but women suffered more anogen- Zostavax, a 14-fold concentrated version answers that patients often ask about the shouldn’t receive the vaccine, said Dr. ital malignancies in the countries where the of Varivax, the varicella zoster vaccine to vaccines at the annual meeting of the Cal- Tyring, professor of dermatology at the vaccine was studied, so they were includ- prevent chicken pox in children, was ap- ifornia Society of Dermatology and Der- University of Texas Health Sciences Cen- ed in the trials, he said.