Differences in the Concentration of Anti-SARS-Cov-2 Igg Antibodies Post-COVID-19 Recovery Or Post-Vaccination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19 Natural Immunity
COVID-19 natural immunity Scientific brief 10 May 2021 Key Messages: • Within 4 weeks following infection, 90-99% of individuals infected with the SARS-CoV-2 virus develop detectable neutralizing antibodies. • The strength and duration of the immune responses to SARS-CoV-2 are not completely understood and currently available data suggests that it varies by age and the severity of symptoms. Available scientific data suggests that in most people immune responses remain robust and protective against reinfection for at least 6-8 months after infection (the longest follow up with strong scientific evidence is currently approximately 8 months). • Some variant SARS-CoV-2 viruses with key changes in the spike protein have a reduced susceptibility to neutralization by antibodies in the blood. While neutralizing antibodies mainly target the spike protein, cellular immunity elicited by natural infection also target other viral proteins, which tend to be more conserved across variants than the spike protein. The ability of emerging virus variants (variants of interest and variants of concern) to evade immune responses is under investigation by researchers around the world. • There are many available serologic assays that measure the antibody response to SARS-CoV-2 infection, but at the present time, the correlates of protection are not well understood. Objective of the scientific brief This scientific brief replaces the WHO Scientific Brief entitled “’Immunity passports’ in the context of COVID-19”, published 24 April 2020.1 This update is focused on what is currently understood about SARS-CoV-2 immunity from natural infection. More information about considerations on vaccine certificates or “passports”will be covered in an update of WHO interim guidance, as requested by the COVID-19 emergency committee.2 Methods A rapid review on the subject was undertaken and scientific journals were regularly screened for articles on COVID-19 immunity to ensure to include all large and robust studies available in the literature at the time of writing. -

Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP)
Morbidity and Mortality Weekly Report Recommendations and Reports February 8, 2002 / Vol. 51 / No. RR-2 General Recommendations on Immunization Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) INSIDE: Continuing Education Examination Centers for Disease Control and Prevention SAFER • HEALTHIER • PEOPLETM MMWR CONTENTS The MMWR series of publications is published by the Introduction ......................................................................... 1 Epidemiology Program Office, Centers for Disease Timing and Spacing of Immunobiologics .............................. 2 General Principles for Vaccine Scheduling ......................... 2 Control and Prevention (CDC), U.S. Department of Spacing of Multiple Doses of the Same Antigen ................ 2 Health and Human Services, Atlanta, GA 30333. Simultaneous Administration ............................................ 4 Nonsimultaneous Administration ...................................... 5 Spacing of Antibody-Containing Products and Vaccines ..... 6 SUGGESTED CITATION Interchangeability of Vaccines from Different Manufacturers 8 Centers for Disease Control and Prevention. General Lapsed Vaccination Schedule ............................................ 8 recommendations on immunization: recom- Unknown or Uncertain Vaccination Status ......................... 8 mendations of the Advisory Committee on Contraindications and Precautions ....................................... 8 Immunization Practices and the -

Systematic Booster After Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure
Article Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure Perrine Parize 1,*, Jérémie Sommé 2 , Laura Schaeffer 3, Florence Ribadeau-Dumas 1, Sheherazade Benabdelkader 1, Agnès Durand 4, Arnaud Tarantola 1, Johann Cailhol 5, Julia Goesch 5, Lauriane Kergoat 1 , Anne-Sophie Le Guern 6, Marie-Laurence Mousel 2, Laurent Dacheux 1 , Paul-Henri Consigny 5, Arnaud Fontanet 3,7, Beata Francuz 2 and Hervé Bourhy 1 1 Institut Pasteur, Unit Lyssavirus Epidemiology and Neuropathology, National Reference Center for Rabies and WHO Collaborating Centre for Reference and Research on Rabies, 75015 Paris, France; fl[email protected] (F.R.-D.); [email protected] (S.B.); [email protected] (A.T.); [email protected] (L.K.); [email protected] (L.D.); [email protected] (H.B.) 2 Institut Pasteur, Occupational Health Department, 75015 Paris, France; [email protected] (J.S.); [email protected] (M.-L.M.); [email protected] (B.F.) 3 Institut Pasteur, Emerging Diseases Epidemiology Unit, Centre for Global Health Research and Education, 75015 Paris, France; [email protected] (L.S.); [email protected] (A.F.) 4 Laboratoire Cerballiance, 75017 Paris, France; [email protected] 5 Citation: Parize, P.; Sommé, J.; Institut Pasteur, Centre Médical, Centre d’Infectiologie Necker-Pasteur, 75015 Paris, France; Schaeffer, L.; Ribadeau-Dumas, F.; [email protected] (J.C.); [email protected] (J.G.); [email protected] (P.-H.C.) 6 Benabdelkader, S.; Durand, A.; Institut Pasteur, Laboratory of the Medical Center, 75015 Paris, France; [email protected] 7 Conservatoire National des Arts et Métiers, 75003 Paris, France Tarantola, A.; Cailhol, J.; Goesch, J.; * Correspondence: [email protected]; Tel.: +33-140-613-436 Kergoat, L.; et al. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -

Outbreak of SARS-Cov-2 Infections, Including COVID-19 Vaccine
Morbidity and Mortality Weekly Report Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings — Barnstable County, Massachusetts, July 2021 Catherine M. Brown, DVM1; Johanna Vostok, MPH1; Hillary Johnson, MHS1; Meagan Burns, MPH1; Radhika Gharpure, DVM2; Samira Sami, DrPH2; Rebecca T. Sabo, MPH2; Noemi Hall, PhD2; Anne Foreman, PhD2; Petra L. Schubert, MPH1; Glen R. Gallagher PhD1; Timelia Fink1; Lawrence C. Madoff, MD1; Stacey B. Gabriel, PhD3; Bronwyn MacInnis, PhD3; Daniel J. Park, PhD3; Katherine J. Siddle, PhD3; Vaira Harik, MS4; Deirdre Arvidson, MSN4; Taylor Brock-Fisher, MSc5; Molly Dunn, DVM5; Amanda Kearns5; A. Scott Laney, PhD2 On July 30, 2021, this report was posted as an MMWR Early Massachusetts, that attracted thousands of tourists from across Release on the MMWR website (https://www.cdc.gov/mmwr). the United States. Beginning July 10, the Massachusetts During July 2021, 469 cases of COVID-19 associated Department of Public Health (MA DPH) received reports of with multiple summer events and large public gatherings in an increase in COVID-19 cases among persons who reside in a town in Barnstable County, Massachusetts, were identified or recently visited Barnstable County, including in fully vac- among Massachusetts residents; vaccination coverage among cinated persons. Persons with COVID-19 reported attending eligible Massachusetts residents was 69%. Approximately densely packed indoor and outdoor events at venues that three quarters (346; 74%) of cases occurred in fully vac- included bars, restaurants, guest houses, and rental homes. On cinated persons (those who had completed a 2-dose course July 3, MA DPH had reported a 14-day average COVID-19 of mRNA vaccine [Pfizer-BioNTech or Moderna] or had incidence of zero cases per 100,000 persons per day in residents received a single dose of Janssen [Johnson & Johnson] vac- of the town in Barnstable County; by July 17, the 14-day cine ≥14 days before exposure). -

Prospecting for an HIV Vaccine D
Brett-Major et al. Tropical Diseases, Travel Medicine and Vaccines (2017) 3:6 DOI 10.1186/s40794-017-0050-4 REVIEW Open Access Prospecting for an HIV vaccine D. M. Brett-Major1,2*, T. A. Crowell1,2 and N. L. Michael1 Abstract Human immunodeficiency virus (HIV) sets several challenges for the development of a preventative HIV vaccine. Predictable, protective natural immunity against HIV does not occur and so unlike most other diseases for which vaccines exist, there are few guideposts from natural infection. Nonetheless, six vaccine efficacy trials have occurred. One in particular, the Thai trial called RV144, showed partial protective efficacy and potential ways ahead to a better vaccine approach. This coupled with other lessons from studies of acute infections as well as an increasingly complex knowledge of HIV-related vaccine immunology bring hope that a vaccine solution might be reached for this pervasive and deadly pandemic. Keywords: Human immunodeficiency virus vaccine protective efficacy immunology review Background Why not already Human immunodeficiency virus (HIV) disease remains A reasonable person new to the global conversation one of the greatest threats to global public health. about HIV might ask, if an HIV vaccine is so critical and According to the World Health Organization (WHO), in the pandemic known for three decades, why do we not 2014 over one million people died from HIV, nearly already have an HIV vaccine? There is no simple answer thirty-seven million people had chronic infection and to this question, though an easy one is that people do two million people newly acquired infections [1]. Of not develop natural, protective immunity to HIV infec- those persons known to be HIV infected, only 35% tion and disease. -

The Model of “Informed Refusal” for Vaccination: How to Fight Against Anti-Vaccinationist Misinformation Without Disregarding the Principle of Self-Determination
Communication The Model of “Informed Refusal” for Vaccination: How to Fight against Anti-Vaccinationist Misinformation without Disregarding the Principle of Self-Determination Stefano D’Errico 1, Emanuela Turillazzi 2, Martina Zanon 1, Rocco Valerio Viola 3, Paola Frati 3,4 and Vittorio Fineschi 3,4,* 1 Department of Surgery, Medicine and Health, University of Trieste, 34149 Trieste, Italy; [email protected] (S.D.); [email protected] (M.Z.) 2 Department of Surgical Pathology, Medical, Molecular and Critical Area, Institute of Legal Medicine, University of Pisa, 56126 Pisa, Italy; [email protected] 3 Department of Anatomical, Histological, Forensic and Orthopaedic Sciences, Sapienza University of Rome, Viale Regina Elena 336, 00161 Rome, Italy; [email protected] (R.V.V.); [email protected] (P.F.) 4 IRCCS (Istituto di Ricerca e Cura a Carattere Scientifico) Neuromed Mediterranean Neurological Institute, Via Atinense 18, 86077 Pozzilli, Italy * Correspondence: [email protected]; Tel.: +39 06 49912722 Abstract: Vaccines are arguably a public health success story as well as an incredibly cost-effective medical resource. Despite this, worldwide concerns about their safety are growing, with the risk of Citation: D’Errico, S.; Turillazzi, E.; increased morbidity and mortality in vaccine-preventable diseases because of vaccine refusal. The Zanon, M.; Viola, R.V.; Frati, P.; global political trend in developed countries is to increasingly reduce mandates and the compulsory Fineschi, V. The Model of “Informed nature of vaccination programs. This is due to strong opposition from anti-vaccination movements Refusal” For Vaccination: How to and groups. While these have existed since the beginnings of vaccinology, they have recently gained Fight Against Anti-Vaccinationist a strong foothold through massive exploitation of the media and especially the internet. -

Statements Contained in This Release As the Result of New Information Or Future Events Or Developments
Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant NEW YORK and MAINZ, GERMANY, July 8, 2021 — As part of Pfizer’s and BioNTech’s continued efforts to stay ahead of the virus causing COVID-19 and circulating mutations, the companies are providing an update on their comprehensive booster strategy. Pfizer and BioNTech have seen encouraging data in the ongoing booster trial of a third dose of the current BNT162b2 vaccine. Initial data from the study demonstrate that a booster dose given 6 months after the second dose has a consistent tolerability profile while eliciting high neutralization titers against the wild type and the Beta variant, which are 5 to 10 times higher than after two primary doses. The companies expect to publish more definitive data soon as well as in a peer-reviewed journal and plan to submit the data to the FDA, EMA and other regulatory authorities in the coming weeks. In addition, data from a recent Nature paper demonstrate that immune sera obtained shortly after dose 2 of the primary two dose series of BNT162b2 have strong neutralization titers against the Delta variant (B.1.617.2 lineage) in laboratory tests. The companies anticipate that a third dose will boost those antibody titers even higher, similar to how the third dose performs for the Beta variant (B.1.351). Pfizer and BioNTech are conducting preclinical and clinical tests to confirm this hypothesis. While Pfizer and BioNTech believe a third dose of BNT162b2 has the potential to preserve the highest levels of protective efficacy against all currently known variants including Delta, the companies are remaining vigilant and are developing an updated version of the Pfizer-BioNTech COVID-19 vaccine that targets the full spike protein of the Delta variant. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -
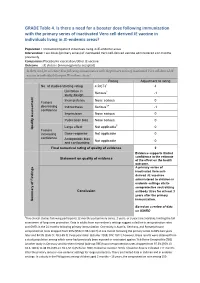
GRADE Table 4. Is There a Need for a Booster Dose Following Immunization Wi
GRADE Table 4. Is there a need for a booster dose following immunization with the primary series of inactivated Vero cell-derived JE vaccine in individuals living in JE-endemic areas? Population : Immunocompetent individuals living in JE-endemic areas Intervention: Two doses (primary series) of inactivated Vero cell-derived vaccine administered ≥12 months previously Comparison: Placebo/no vaccination/other JE vaccine Outcome : JE disease (immunogenicity accepted) Is there need for a booster dose following immunization with the primary series of inactivated Vero cell-derived JE vaccine in individuals living in JE-endemic areas? Rating Adjustment to rating No. of studies/starting rating 4 RCTs1 4 Limitation in 2 Serious -1 study design Inconsistency None serious 0 Factors decreasing Indirectness Serious3,4 -1 confidence Imprecision None serious 0 Publication bias None serious 0 Large effect Not applicable5 0 Quality Assessment Quality Factors increasing Dose-response Not applicable 0 confidence Antagonistic bias Not applicable 0 and confounding Final numerical rating of quality of evidence 2 Evidence supports limited confidence in the estimate Statement on quality of evidence of the effect on the health outcome. A primary series of inactivated Vero cell- derived JE vaccines administered to children in endemic settings elicits seroprotective neutralizing Conclusion antibody titres for at least 3 years after the primary immunization. Summary of Findings of Summary Based on a review of data on IXIARO 1Five clinical studies following participants 12 months post-primary series, 2 years, or 3 years are available, limiting the full assessment of long-term protection. Data in adults from non-endemic settings suggest a decline in seroprotection rates and GMTs in the 24 months following primary immunization. -

SARS-Cov-2 Vaccine Breakthrough Infections Are Asymptomatic Or Mildly Symptomatic and Are Infrequently Transmitted
medRxiv preprint doi: https://doi.org/10.1101/2021.06.29.21259500; this version posted July 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC 4.0 International license . SARS-CoV-2 vaccine breakthrough infections are asymptomatic or mildly symptomatic and are infrequently transmitted. Francesca Rovida1, Irene Cassaniti1, Stefania Paolucci1, Elena Percivalle1, Antonella Sarasini1, Antonio Piralla1, Federica Giardina1, Jose Camilla Sammartino1, Alessandro Ferrari1, Federica Bergami1, Alba Muzzi2, Viola Novelli2, Alessandro Meloni2,6, Anna Maria Grugnetti3, Giuseppina Grugnetti3, Claudia Rona2, Marinella Daglio2, Carlo Marena2, Antonio Triarico4, Daniele Lilleri1*, Fausto Baldanti1,5 1Molecular Virology Unit, Microbiology and Virology Department, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 2Medical Direction, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 3Health Professions Direction, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 4Direzione Sanitaria, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 5Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy; 6 Department of Public Health, Experimental and Forensic Medicine, Section of Hygiene, University of Pavia, Pavia, Italy; *Correspondence to: Daniele Lilleri; [email protected] World count: Abstract: 148 Text: 1900 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2021.06.29.21259500; this version posted July 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. -

(ACIP) General Best Guidance for Immunization
Appendix 1: Glossary Adverse event. An undesirable medical condition that occurs following vaccination which might be truly caused by a vaccine, or it might be pure coincidence. Adverse reaction. An undesirable medical condition that has been demonstrated to be caused by a vaccine. Evidence for the causal relation is usually obtained through randomized clinical trials, controlled epidemiologic studies, isolation of the vaccine strain from the pathogenic site, or recurrence of the condition with repeated vaccination (i.e., rechallenge); synonyms include side effect and adverse effect. Adjuvant. A vaccine component distinct from the antigen that enhances the immune response to the antigen. Antitoxin. A solution of antibodies against a toxin. Antitoxin can be derived from either human (e.g., tetanus immune globulin) or animal (usually equine) sources (e.g., diphtheria and botulism antitoxin). Antitoxins are used to confer passive immunity and for treatment. Hyperimmune globulin (specific). Special preparations obtained from blood plasma from donor pools preselected for a high antibody content against a specific antigen (e.g., hepatitis B immune globulin, varicella-zoster immune globulin, rabies immune globulin, tetanus immune globulin, vaccinia immune globulin, cytomegalovirus immune globulin, botulism immune globulin). Immune globulin. A sterile solution containing antibodies, which are usually obtained from human blood. It is obtained by cold ethanol fractionation of large pools of blood plasma and contains 15%-18% protein. Intended for intramuscular administration, immune globulin is primarily indicated for routine maintenance of immunity among certain immunodeficient persons and for passive protection against measles and hepatitis A. General Best Practice Guidelines for Immunization: Appendix 1: Glossary 189 Immunobiologic. Antigenic substances (e.g., vaccines and toxoids) or antibody- containing preparations (e.g., globulins and antitoxins) from human or animal donors.