Developing a Vaccine Against HIV Infection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adverse Reactions to HIV Vaccines: Medical, Ethical, and Legal Issues
Adverse Reactions to HIV Vaccines: Medical, Ethical, and Legal Issues September 1995 OTA-BP-H-163 GPO stock #052-003-01429-7 Recommended Citation: U.S. Congress, Office of Technology Assessment, Adverse Reactions to HIV Vaccines: Medical, Ethical, and Legal Issues, OTA-BP-H-163 (Washington, DC: U.S. Government Printing Office, September 1995). oreword IDS researchers are investigating new vaccines that would prevent infection with HIV and reduce the spread of AIDS. Some have argued that product liabil- ity concerns have discouraged investment in HIV vaccine research and devel- opment. The purpose of this OTA background paper is to describe the current state of development of HIV vaccines, and to discuss what is known about adverse reac- tions that may occur. The background paper provides an overview of ethical issues that arise in the conduct of HIV vaccine trials. The report also discusses alternatives to the current product liability system to encourage the development of HIV vaccines and to fairly compensate those who are harmed as a result of adverse reactions to the vaccine. This background paper was prepared in response to a request from the Subcommittee on Health of the House Ways and Means Committee. It is eleventh in OTA’s series of studies on HIV-related issues. The preceding papers in this series were: Do Insects Transmit AIDS? (9/87), AIDS and Health Insurance: An OTA Survey (2/88), How Effective is AIDS Education? (6/88), The Impact of AIDS on the Kaiser Permanente Medical Care Program (Northern California Region) (7/88), How Has Federal Research on AIDS/HIV Disease Contributed to Other Fields? (4/90), The Effectiveness of Drug Abuse Treatment: Implications for Controlling AIDS/HIV Infection (9/90), HIV in the Health Care Workplace (11/91), The CDC’s Case Definition of AIDS: Implications of the Proposed Revisions (8/92), Difficult-to-reuse Needles for the Prevention of HIV Infection Among Injecting Drug Abusers (10/92), and External Review of the Federal Centers for Disease Control and Prevention’s HIV Prevention Programs (9/94). -

Systematic Booster After Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure
Article Systematic Booster after Rabies Pre-Exposure Prophylaxis to Alleviate Rabies Antibody Monitoring in Individuals at Risk of Occupational Exposure Perrine Parize 1,*, Jérémie Sommé 2 , Laura Schaeffer 3, Florence Ribadeau-Dumas 1, Sheherazade Benabdelkader 1, Agnès Durand 4, Arnaud Tarantola 1, Johann Cailhol 5, Julia Goesch 5, Lauriane Kergoat 1 , Anne-Sophie Le Guern 6, Marie-Laurence Mousel 2, Laurent Dacheux 1 , Paul-Henri Consigny 5, Arnaud Fontanet 3,7, Beata Francuz 2 and Hervé Bourhy 1 1 Institut Pasteur, Unit Lyssavirus Epidemiology and Neuropathology, National Reference Center for Rabies and WHO Collaborating Centre for Reference and Research on Rabies, 75015 Paris, France; fl[email protected] (F.R.-D.); [email protected] (S.B.); [email protected] (A.T.); [email protected] (L.K.); [email protected] (L.D.); [email protected] (H.B.) 2 Institut Pasteur, Occupational Health Department, 75015 Paris, France; [email protected] (J.S.); [email protected] (M.-L.M.); [email protected] (B.F.) 3 Institut Pasteur, Emerging Diseases Epidemiology Unit, Centre for Global Health Research and Education, 75015 Paris, France; [email protected] (L.S.); [email protected] (A.F.) 4 Laboratoire Cerballiance, 75017 Paris, France; [email protected] 5 Citation: Parize, P.; Sommé, J.; Institut Pasteur, Centre Médical, Centre d’Infectiologie Necker-Pasteur, 75015 Paris, France; Schaeffer, L.; Ribadeau-Dumas, F.; [email protected] (J.C.); [email protected] (J.G.); [email protected] (P.-H.C.) 6 Benabdelkader, S.; Durand, A.; Institut Pasteur, Laboratory of the Medical Center, 75015 Paris, France; [email protected] 7 Conservatoire National des Arts et Métiers, 75003 Paris, France Tarantola, A.; Cailhol, J.; Goesch, J.; * Correspondence: [email protected]; Tel.: +33-140-613-436 Kergoat, L.; et al. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -

Coversheet for Thesis in Sussex Research Online
A University of Sussex DPhil thesis Available online via Sussex Research Online: http://eprints.sussex.ac.uk/ This thesis is protected by copyright which belongs to the author. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Please visit Sussex Research Online for more information and further details Knowledge Accumulation and Vaccine Innovation: Lessons from Polio and HIV/AIDS Ohid Yaqub Doctor of Philosophy University of Sussex Submitted in September 2008 ii I hereby declare that this thesis has not been submitted, either in the same or different form, to this or any other university for a degree. Ohid Yaqub iii To my parents and Corinne, Two worlds that should not be separate. iv ACKNOWLEDGEMENTS This thesis was funded by the Economic and Social Research Council and supervised by Paul Nightingale. Paul is a supervisor who is extremely generous with his time, ideas and encouragement; and who manages to make academia look extremely fun. His energy and enthusiasm were most important to me when I really thought the ship was sinking. I cannot thank him enough and feel privileged to have worked with him. My first opportunity to pursue some of the ideas in this thesis was under the supervision of Ed Steinmueller and Aldo Geuna. -

Statements Contained in This Release As the Result of New Information Or Future Events Or Developments
Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant NEW YORK and MAINZ, GERMANY, July 8, 2021 — As part of Pfizer’s and BioNTech’s continued efforts to stay ahead of the virus causing COVID-19 and circulating mutations, the companies are providing an update on their comprehensive booster strategy. Pfizer and BioNTech have seen encouraging data in the ongoing booster trial of a third dose of the current BNT162b2 vaccine. Initial data from the study demonstrate that a booster dose given 6 months after the second dose has a consistent tolerability profile while eliciting high neutralization titers against the wild type and the Beta variant, which are 5 to 10 times higher than after two primary doses. The companies expect to publish more definitive data soon as well as in a peer-reviewed journal and plan to submit the data to the FDA, EMA and other regulatory authorities in the coming weeks. In addition, data from a recent Nature paper demonstrate that immune sera obtained shortly after dose 2 of the primary two dose series of BNT162b2 have strong neutralization titers against the Delta variant (B.1.617.2 lineage) in laboratory tests. The companies anticipate that a third dose will boost those antibody titers even higher, similar to how the third dose performs for the Beta variant (B.1.351). Pfizer and BioNTech are conducting preclinical and clinical tests to confirm this hypothesis. While Pfizer and BioNTech believe a third dose of BNT162b2 has the potential to preserve the highest levels of protective efficacy against all currently known variants including Delta, the companies are remaining vigilant and are developing an updated version of the Pfizer-BioNTech COVID-19 vaccine that targets the full spike protein of the Delta variant. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -
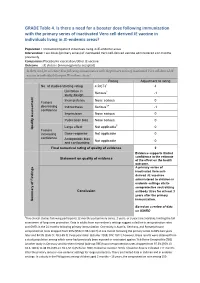
GRADE Table 4. Is There a Need for a Booster Dose Following Immunization Wi
GRADE Table 4. Is there a need for a booster dose following immunization with the primary series of inactivated Vero cell-derived JE vaccine in individuals living in JE-endemic areas? Population : Immunocompetent individuals living in JE-endemic areas Intervention: Two doses (primary series) of inactivated Vero cell-derived vaccine administered ≥12 months previously Comparison: Placebo/no vaccination/other JE vaccine Outcome : JE disease (immunogenicity accepted) Is there need for a booster dose following immunization with the primary series of inactivated Vero cell-derived JE vaccine in individuals living in JE-endemic areas? Rating Adjustment to rating No. of studies/starting rating 4 RCTs1 4 Limitation in 2 Serious -1 study design Inconsistency None serious 0 Factors decreasing Indirectness Serious3,4 -1 confidence Imprecision None serious 0 Publication bias None serious 0 Large effect Not applicable5 0 Quality Assessment Quality Factors increasing Dose-response Not applicable 0 confidence Antagonistic bias Not applicable 0 and confounding Final numerical rating of quality of evidence 2 Evidence supports limited confidence in the estimate Statement on quality of evidence of the effect on the health outcome. A primary series of inactivated Vero cell- derived JE vaccines administered to children in endemic settings elicits seroprotective neutralizing Conclusion antibody titres for at least 3 years after the primary immunization. Summary of Findings of Summary Based on a review of data on IXIARO 1Five clinical studies following participants 12 months post-primary series, 2 years, or 3 years are available, limiting the full assessment of long-term protection. Data in adults from non-endemic settings suggest a decline in seroprotection rates and GMTs in the 24 months following primary immunization. -

Hepatitis B and Healthcare Personnelq &A IAC Answers Frequently Asked Questions About How to Protect Healthcare Personnel
Hepatitis B and Healthcare PersonnelQ &A IAC answers frequently asked questions about how to protect healthcare personnel Experts from the Immunization Action Engerix-B (GSK) or Recombivax HB (Merck) should be vaccinated against hepatitis B if Coalition (IAC) answer your questions may be completed with Heplisav-B. However, they haven’t been previously vaccinated. about hepatitis B (HepB) vaccine. You’ll data are limited on the safety and immunoge- Receipt of the vaccine is not a reason to dis- nicity effects when Heplisav-B is interchanged continue breast-feeding. find additional &Q As about hepatitis B with hepatitis B vaccines from other manufac- There are no clinical studies of Heplisav-B in vaccine on the “Ask the Experts” section turers. When feasible, the same manufactur- pregnant women. Available human data on of immunize.org at www.immunize.org/ er’s vaccines should be used to complete the Heplisav-B administered to pregnant women askexperts/experts_hepb.asp series. However, vaccination should not be are insufficient to assess vaccine-associated deferred when the manufacturer of the previ- risks in pregnancy. Until safety data are avail- ously administered vaccine is unknown or able for Heplisav-B, providers should con- Hepatitis B Vaccination when the vaccine from the same manufac- tinue to vaccinate pregnant women needing turer is unavailable. hepatitis B vaccination with a vaccine from Which people who work in healthcare settings The 2-dose hepatitis B vaccine series only a different manufacturer. need hepatitis B vaccine? applies when both doses in the series consist Is there a recommendation for routine booster The Occupational Safety and Health Adminis- of Heplisav-B. -

Vaccinations for Pregnant Women the Table Below Shows Which Vaccinations You May Or May Not Need During Your Pregnancy
Vaccinations for Pregnant Women The table below shows which vaccinations you may or may not need during your pregnancy. Vaccine Do you need it during your pregnancy? Influenza Yes! You need a flu shot every fall (or even as late as winter or spring) for your protection and for the protection of your baby and others around you. It’s safe to get the vaccine at any time during your pregnancy. Tetanus, diphtheria, Yes! Women who are pregnant need a dose of Tdap vaccine (the adult whooping cough vaccine) during each pregnancy, prefer- whooping cough ably in the early part of the third trimester. It’s safe to be given during pregnancy and will help protect your baby from whooping (pertussis) cough in the first few months after birth when he or she is most vulnerable. After Tdap, you need a Tdap or Td booster dose every Tdap, Td 10 years. Consult your healthcare provider if you haven’t had at least 3 tetanus- and diphtheria-toxoid containing shots some- time in your life or if you have a deep or dirty wound. Human No. This vaccine is not recommended to be given during pregnancy, but if you inadvertently receive it, this is not a cause for papillomavirus concern. HPV vaccine is recommended for all people age 26 or younger, so if you are in this age group, make sure you are HPV vaccinated before or after your pregnancy. People age 27 through 45 may also be vaccinated against HPV after discussion with their healthcare provider. The vaccine is given in 2 or 3 doses (depending on the age at which the first dose is given) over a 6-month period. -

Status of HIV Vaccine Research & Development
Status of HIV Vaccine Research & Development Wayne C. Koff, Ph.D. Chief Scientific Officer, IAVI Global Vaccine Immunization Research Forum Bethesda, Maryland March 4, 2014 HIV continues to devastate…. 35.3 million people living with HIV worldwide 2.3 million new infections in 2012 ; 2012 2008 6,300 new HIV infections daily 33 million 35.3 million 36 million AIDS-related deaths to date 2005 32 million Women bear the brunt of the epidemic, representing almost 60% of HIV-infected adults in Africa and half of adults worldwide 2000 28.5 million Since the beginning >70,000,000 HIV Infections 1995 18.5 million 1990 7.5 million people living with HIV Remarkable scale up of treatment; however, doesn’t solve problem. Lifetime treatment required and for every (1) person put on treatment, (2) are newly infected. THE WORLD NEEDS AN HIV VACCINE Source: Joint United Nations Programme on HIV/AIDS Public Health Impact: A Vaccine Is Needed to “Get Close to Zero” Cumulative infections avoided 2011-50 22.5M 16.0M New HIV Infections HIV New 7.4M * An illustrative vaccine with an assumed efficacy of 60%, not representative of any specific candidate in development. Coverage reaches 70% in generalized HIV/AIDS epidemics, 60% in concentrated epidemics. Potential impact of an AIDS vaccine as part of the UNAIDS Enhanced Investment Framework, IFE Modeling project – UNAIDS, Futures Institute, IAVI, AVAC [funded by USAID] 3 AIDS vaccine development: Scientific Challenges 1. HIV variability 2. Lack of an ideal animal model 3. Natural immunity fails to clear HIV 4. HIV is a retrovirus- integrates into the host genome- short window of opportunity to control 5. -

Vaccine Immunology Claire-Anne Siegrist
2 Vaccine Immunology Claire-Anne Siegrist To generate vaccine-mediated protection is a complex chal- non–antigen-specifc responses possibly leading to allergy, lenge. Currently available vaccines have largely been devel- autoimmunity, or even premature death—are being raised. oped empirically, with little or no understanding of how they Certain “off-targets effects” of vaccines have also been recog- activate the immune system. Their early protective effcacy is nized and call for studies to quantify their impact and identify primarily conferred by the induction of antigen-specifc anti- the mechanisms at play. The objective of this chapter is to bodies (Box 2.1). However, there is more to antibody- extract from the complex and rapidly evolving feld of immu- mediated protection than the peak of vaccine-induced nology the main concepts that are useful to better address antibody titers. The quality of such antibodies (e.g., their these important questions. avidity, specifcity, or neutralizing capacity) has been identi- fed as a determining factor in effcacy. Long-term protection HOW DO VACCINES MEDIATE PROTECTION? requires the persistence of vaccine antibodies above protective thresholds and/or the maintenance of immune memory cells Vaccines protect by inducing effector mechanisms (cells or capable of rapid and effective reactivation with subsequent molecules) capable of rapidly controlling replicating patho- microbial exposure. The determinants of immune memory gens or inactivating their toxic components. Vaccine-induced induction, as well as the relative contribution of persisting immune effectors (Table 2.1) are essentially antibodies— antibodies and of immune memory to protection against spe- produced by B lymphocytes—capable of binding specifcally cifc diseases, are essential parameters of long-term vaccine to a toxin or a pathogen.2 Other potential effectors are cyto- effcacy. -

HIV VACCINE RESEARCH and DEVELOPMENT CD8 Group
GLOSSARY OF KEY HIV VACCINE TERMS Immunogenicity – when attributed to a test vaccine, defines the • Since 2001, USAID has contributed $134 million to help discover an HIV vaccine. Currently, USAID is committing annual product’s ability to cause the body to produce antibodies or T-cells funding of $28 million through 2011 for HIV vaccine R&D. that may protect against an infection, disease, or foreign substance. • USAID provides support for all phases of HIV vaccine applied R&D, infrastructure, and capacity building for clinical trial Innate Immunity – a relatively nonspecific response that protects conduct, public communications, and policy analysis through a partnership with the International AIDS Vaccine Initiative. against a whole class or type of invaders but does not generate USAID does not support basic research. TECHNICAL ISSUE BRIEF immune memory (see adaptive immune response). Killer T-cells – a group of T-cells that is activated by helper T-cells • USAID plans involvement with the Global HIV/AIDS Vaccine Enterprise. PATHWAYS OF DISCOVERY: and has the ability to destroy cells infected by foreign invaders (such as viruses). Also known as cytotoxic T-cells, they may belong to the • USAID facilitates coordination between HIV vaccine clinical trial activities and HIV/AIDS prevention, care, and treatment HIV VACCINE RESEARCH AND DEVELOPMENT CD8 group. programs in developing countries. Lymphocytes – the diverse set of white blood cells (each with different functions) that are responsible for immune responses. virologists convenes twice a year to review the R&D portfolio activities. As the Global HIV/AIDS Vaccine Enterprise evolves Introduction There are two main types: B-cells (responsible for producing anti- under consideration by the organization.