Taxa Common Size Class Size Category (Cm) Number of Stomachs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
HELCOM Red List
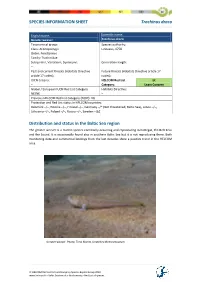
SPECIES INFORMATION SHEET Trachinus draco English name: Scientific name: Greater weever Trachinus draco Taxonomical group: Species authority: Class: Actinopterygii Linnaeus, 1758 Order: Perciformes Family: Trachinidae Subspecies, Variations, Synonyms: Generation length: – Past and current threats (Habitats Directive Future threats (Habitats Directive article 17 article 17 codes): – codes): – IUCN Criteria: HELCOM Red List LC – Category: Least Concern Global / European IUCN Red List Category Habitats Directive: NE/NE – Previous HELCOM Red List Category (2007): VU Protection and Red List status in HELCOM countries: Denmark –/–, Estonia –/–, Finland –/–, Germany –/* (Not threatened, Baltic Sea), Latvia –/–, Lithuania –/–, Poland –/–, Russia –/–, Sweden –/LC Distribution and status in the Baltic Sea region The greater weever is a marine species commonly occurring and reproducing in Kattegat, the Belt Seas and the Sound. It is occasionally found also in southern Baltic Sea but it is not reproducing there. Both monitoring data and commercial landings from the last decades show a positive trend in the HELCOM area. Greater weaver. Photo: Timo Moritz, Deutches Meeresmuseum. © HELCOM Red List Fish and Lamprey Species Expert Group 2013 www.helcom.fi > Baltic Sea trends > Biodiversity > Red List of species SPECIES INFORMATION SHEET Trachinus draco Fig.1 Catch per unit effort (number per trawling hour) of greater weever in international bottom trawl surveys in Kattegat (IBTS) during third quarter of the year (Linear fit and correlation coefficient -

Diet Composition of Cod (Gadus Morhua): Small-Scale Differences in a Sub-Arctic Fjord
Diet composition of cod (Gadus morhua): small-scale differences in a sub-arctic fjord Enoksen, Siri Elise BI309F MSc IN MARINE ECOLOGY Faculty for Biosciences and Aquaculture May 2015 Acknowledgements The presented thesis is the final part of a two-year Master of Science program at the Faculty of Biosciences and Aquaculture, University of Nordland, Bodø, Norway. I owe my supervisor Associate Professor Henning Reiss eternal gratitude for his patience and all the help with sampling, species determination and writing of this thesis. Without his expertise and guidance, this master thesis would not have been possible. A special thanks to Bjørn Tore Zahl at Saltstraumen Brygge, Geir Jøran Nyheim at Saltstraumen camping, Lill-Anita Stenersen at Kafe Kjelen, Fauske Båtforening and Saltdal Båtforening for helping during sampling, Coop Extra Bygg Fauske for sponsoring sheds for collecting stations, and to all anglers who handed inn samples. This project would not have been possible without their help. I would like to thank Professor Truls Moum, Martina Kopp, Vigdis Edvardsen, Tor Erik Jørgensen and Teshome Tilahun Bizuayehu for help and guidance during DNA barcoding analysis. I thank Nina Tande Hansen and Bibbi Myrvoll at Karrieresenteret Indre Salten for believing in me and convincing me that I was capable of studying at university level. This thesis would not have been possible without their guidance. I would also like to thank my family for their patience during the five years of fulfilling my Master. i Table of contents Acknowledgements ......................................................................................................................... -

Scenario Calculations of Mercury Exposure
VKM Report 2019:3 Scenario calculations of mercury exposure from fish and overview of species with high mercury concentrations Opinion of the Panel on Contaminants of the Norwegian Scientific Committee for Food and Environment Report from the Norwegian Scientific Committee for Food and Environment (VKM) 2019:3 Scenario calculations of mercury exposure from fish and overview of species with high mercury concentrations Opinion of the Panel on Contaminants of the Norwegian Scientific Committee for Food and Environment 05.04.2019 ISBN: 978-82-8259-319-9 ISSN: 2535-4019 Norwegian Scientific Committee for Food and Environment (VKM) Po 4404 Nydalen N – 0403 Oslo Norway Phone: +47 21 62 28 00 Email: [email protected] vkm.no vkm.no/english Cover photo: Colourbox Suggested citation: VKM, Heidi Amlund, Kirsten Eline Rakkestad, Anders Ruus, Jostein Starrfelt, Jonny Beyer, Anne Lise Brantsæter, Sara Bremer, Gunnar Sundstøl Eriksen, Espen Mariussen, Ingunn Anita Samdal, Cathrine Thomsen and Helle Katrine Knutsen (2019). Scenario calculations of mercury exposure from fish and overview of species with high mercury concentrations. Opinion of the Panel on Contaminants of the Norwegian Scientific Committee for Food and Environment. VKM report 2019:3, ISBN: 978-82-8259-319-9, ISSN: 2535-4019. Norwegian Scientific Committee for Food and Environment (VKM), Oslo, Norway. Scenario calculations of mercury exposure from fish and overview of species with high mercury concentrations Preparation of the opinion The Norwegian Scientific Committee for Food and Environment (Vitenskapskomiteen for mat og miljø, VKM) appointed a project group to answer the request from the Norwegian Food Safety Authority. The project group consisted of three VKM-members, and three employees, including a project leader, from the VKM secretariat. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Atlas of North Sea Fishes
ICES COOPERATIVE RESEARCH REPORT RAPPORT DES RECHERCHES COLLECTIVES NO. 194 Atlas of North Sea Fishes Based on bottom-trawl survey data for the years 1985—1987 Ruud J. Knijn1, Trevor W. Boon2, Henk J. L. Heessen1, and John R. G. Hislop3 'Netherlands Institute for Fisheries Research, Haringkade 1, PO Box 6 8 , 1970 AB Umuiden, The Netherlands 2MAFF, Fisheries Laboratory, Lowestoft, Suffolk NR33 OHT, England 3Marine Laboratory, PO Box 101, Victoria Road, Aberdeen AB9 8 DB, Scotland Fish illustrations by Peter Stebbing International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer Palægade 2—4, DK-1261 Copenhagen K, Denmark September 1993 Copyright ® 1993 All rights reserved No part of this book may be reproduced in any form by photostat or microfilm or stored in a storage system or retrieval system or by any other means without written permission from the authors and the International Council for the Exploration of the Sea Illustrations ® 1993 Peter Stebbing Published with financial support from the Directorate-General for Fisheries, AIR Programme, of the Commission of the European Communities ICES Cooperative Research Report No. 194 Atlas of North Sea Fishes ISSN 1017-6195 Printed in Denmark Contents 1. Introduction............................................................................................................... 1 2. Recruit surveys.................................................................................. 3 2.1 General purpose of the surveys..................................................................... -

Marine Fishes from Galicia (NW Spain): an Updated Checklist
1 2 Marine fishes from Galicia (NW Spain): an updated checklist 3 4 5 RAFAEL BAÑON1, DAVID VILLEGAS-RÍOS2, ALBERTO SERRANO3, 6 GONZALO MUCIENTES2,4 & JUAN CARLOS ARRONTE3 7 8 9 10 1 Servizo de Planificación, Dirección Xeral de Recursos Mariños, Consellería de Pesca 11 e Asuntos Marítimos, Rúa do Valiño 63-65, 15703 Santiago de Compostela, Spain. E- 12 mail: [email protected] 13 2 CSIC. Instituto de Investigaciones Marinas. Eduardo Cabello 6, 36208 Vigo 14 (Pontevedra), Spain. E-mail: [email protected] (D. V-R); [email protected] 15 (G.M.). 16 3 Instituto Español de Oceanografía, C.O. de Santander, Santander, Spain. E-mail: 17 [email protected] (A.S); [email protected] (J.-C. A). 18 4Centro Tecnológico del Mar, CETMAR. Eduardo Cabello s.n., 36208. Vigo 19 (Pontevedra), Spain. 20 21 Abstract 22 23 An annotated checklist of the marine fishes from Galician waters is presented. The list 24 is based on historical literature records and new revisions. The ichthyofauna list is 25 composed by 397 species very diversified in 2 superclass, 3 class, 35 orders, 139 1 1 families and 288 genus. The order Perciformes is the most diverse one with 37 families, 2 91 genus and 135 species. Gobiidae (19 species) and Sparidae (19 species) are the 3 richest families. Biogeographically, the Lusitanian group includes 203 species (51.1%), 4 followed by 149 species of the Atlantic (37.5%), then 28 of the Boreal (7.1%), and 17 5 of the African (4.3%) groups. We have recognized 41 new records, and 3 other records 6 have been identified as doubtful. -

ICES CH 1986/G:58 Ref. Pelagic Fish Committee FISH COHHUNITIES OF
ICES C.H. 1986/G:58 Ref. Pelagic Fish Committee FISH COHHUNITIES OF THE NORWEGIAN OEEPS: SPEelES COHPOSITION ANO OISTRIBUTIONAl PATTERNS. by Odd Aksel Bergstad Department of fisheries biology. University of Bergen. Norway. \ P.O.Bo~ 1839. N-5011 Bergen-Nordnes / ABSTRACT Preliminary results from studies of the fishes inhabiting the Norwegian Oeeps and bordering slopes are presented. including accounts of species composition and distributional patterns. The Norwegian Oeeps proper is a nursery and feeding area for a number of species. mainly species which are abundant along the upper continental slopes of the North Atlantic and the deep fjord environments. The more abundant include the greater argentine. Argentina silus. blue whiting. Hicromesistius poutassou. and in the Skagerrak. roundnose grenadier. Coryphaenoides rupestris. Above the 200m isobath the species composition resembles the one found in adjacent coastal areas or on the North Sea plateau. The more abundant demersal species are Norway pout. Trisopterus esmarkii. saithe, Pollachius virens, and haddock, Helanogrammus aeglefinus. Data from hydroacoustic surveys and bottom trawl surveys suggest pronounced seasonal changes in distributional patterns of some species, notably the greater argentine and blue whiting. • INTRODUCTIOH The Norwegian Deeps, with depths in the range 275-700 m, represents a major topographical feature of the comparatively shallow and even North Sea (Fig.1). As such, it has a pronounced influence on watermass distributions and current patterns in the eastern Horth Sea, the Skagerrak and in the Norwegian Coastal waters (Furnes et al. 1986). From fishery data and a few regional studies it is known that the Horwegian Deeps is inhabited by a .fish fauna differing rather markedly from the one found in the bordering shallow waters (Hjort and Ruud 1938; Sahrhage 1964, Aker et Al. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

First Detection of Piscine Reovirus (PRV) in Marine Fish Species
Vol. 97: 255–258, 2012 DISEASES OF AQUATIC ORGANISMS Published January 24 doi: 10.3354/dao02425 Dis Aquat Org NOTE First detection of piscine reovirus (PRV) in marine fish species Christer R. Wiik-Nielsen1,*, Marie Løvoll1, Nina Sandlund,2 Randi Faller1, Jannicke Wiik-Nielsen1, Britt Bang Jensen1 1Norwegian Veterinary Institute, PO Box 750 Sentrum, 0106 Oslo, Norway 2Institute of Marine Research, PO Box 1870 Nordnes, 5817 Bergen, Norway ABSTRACT: Heart and skeletal muscle inflammation (HSMI) is a disease that affects farmed Atlantic salmon Salmo salar L. several months after the fish have been transferred to seawater. Recently, a new virus called piscine reovirus (PRV) was identified in Atlantic salmon from an out- break of HSMI and in experimentally challenged fish. PRV is associated with the development of HSMI, and has until now only been detected in Atlantic salmon. This study investigates whether the virus is also present in wild fish populations that may serve as vectors for the virus. The virus was found in few of the analyzed samples so there is probably a more complex relationship that involves several carriers and virus reservoirs. KEY WORDS: Heart and skeletal muscle inflammation · HSMI · Transmission · Reovirus · Wild marine fish species · Farmed fish · PCR Resale or republication not permitted without written consent of the publisher INTRODUCTION stranded RNA virus related to the Reoviridae group, named PRV (Palacios et al. 2010). PRV is detected in A severe disease affecting farmed Atlantic salmon both farmed and wild Atlantic salmon. In wild Salmo salar L. in Norway is heart and skeletal muscle Atlantic salmon PRV has only been detected in low inflammation (HSMI). -

European Trawlers Are Destroying the Oceans
EUROPEAN TRAWLERS ARE DESTROYING THE OCEANS Introduction Nearly 100,000 vessels make up the European Union fishing fleet. This includes boats that fish both in EU waters (the domestic fleet), in the waters of other countries and in international waters (the deep-sea fleet). In addition, there is an unknown number of vessels belonging to other European countries that are not members of the EU which could approach a figure half that of the EU fleet. The majority of these vessels sail under the flag of a European country but there are also boats, particularly those fishing on the high seas, which despite being managed, chartered or part owned by European companies, use the flag of the country where they catch their fish or sail under flags of convenience (FOCs). The Fisheries Commission has called for a reform of the Common Fisheries Policy (CFP) to achieve a reduction of 40% in the EU fishing capacity, as forecasts show that by simply following the approved multi-annual plans, barely 8.5% of vessels and 18% of gross tonnage would be decommissioned1; an achievement very distant from scientific recommendations. Moreover, from among these almost 100,000 vessels, the EU is home to a particularly damaging fleet: the 15,000 trawlers that operate in European waters, as well as those of third countries or those fishing on the high seas. These trawlers are overexploiting marine resources and irreversibly damaging some of the most productive and biodiverse ecosystems on the planet. The 40% reduction called for by the Commission could be easily achieved if the primary objective of this proposal was focused both on eliminating the most destructive fishing techniques and reducing fishing overcapacity. -

Length-Weight Relationships of Marine Fish Collected from Around the British Isles
Science Series Technical Report no. 150 Length-weight relationships of marine fish collected from around the British Isles J. F. Silva, J. R. Ellis and R. A. Ayers Science Series Technical Report no. 150 Length-weight relationships of marine fish collected from around the British Isles J. F. Silva, J. R. Ellis and R. A. Ayers This report should be cited as: Silva J. F., Ellis J. R. and Ayers R. A. 2013. Length-weight relationships of marine fish collected from around the British Isles. Sci. Ser. Tech. Rep., Cefas Lowestoft, 150: 109 pp. Additional copies can be obtained from Cefas by e-mailing a request to [email protected] or downloading from the Cefas website www.cefas.defra.gov.uk. © Crown copyright, 2013 This publication (excluding the logos) may be re-used free of charge in any format or medium for research for non-commercial purposes, private study or for internal circulation within an organisation. This is subject to it being re-used accurately and not used in a misleading context. The material must be acknowledged as Crown copyright and the title of the publication specified. This publication is also available at www.cefas.defra.gov.uk For any other use of this material please apply for a Click-Use Licence for core material at www.hmso.gov.uk/copyright/licences/ core/core_licence.htm, or by writing to: HMSO’s Licensing Division St Clements House 2-16 Colegate Norwich NR3 1BQ Fax: 01603 723000 E-mail: [email protected] 3 Contents Contents 1. Introduction 5 2. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste