Gamma Knife Radiosurgery for Locally Recurrent Choroidal Melanoma Following Plaque Radiotherapy Osama A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Combined Therapy in the Treatment of Retinopathy and Optic Neuropathy Due to Radiotherapy in the Uveal Melanoma
Published online: 2020-01-16 THIEME Original Article 1 The Role of Combined Therapy in the Treatment of Retinopathy and Optic Neuropathy Due to Radiotherapy in the Uveal Melanoma Yasemin Benderli Cihan1 1Department of Radiation Oncology, Kayseri Education and Address for correspondence Yasemin Benderli Cihan, MD, Department Research Hospital, Kayseri, Turkey of Radiation Oncology, Kayseri Education and Research Hospital, Sanayi District, Ataturk Boulevard, Hastane Street, No 78, 38010 Kocasinan, Kayseri, Turkey (e-mail: [email protected]). Asian J Oncol 2020;6:1–2 Abstract Introduction Uveal melanoma has a relatively low incidence. Transpupillary thermo- therapy (TTT), hypofractioned stereotactic radiotherapy (RT), stereotactic radiosur- gery, plaque brachytherapy, charged particle radiation therapy, local tumor resection, enucleation, and exantation are applied in the treatment. Methods The importance given to radiotherapy has increased to get more satisfac- tory results while treating the patient. However, it is the treatment of radiation reti- nopathy and optic neuropathy from complications. Results Radiation retinopathy and optic neuropathy are the most important compli- Keywords cations related to radiotherapy in the treatment of uveal melanoma. In recent years, ► uveal melanoma many studies have been performed on the treatment of radiation retinopathy and ► radiotherapy optic neuropathy. ► retinopathy Conclusion The consecutive use of triamcinolone in combination with anti-VEGF ► optic neuropathy supports that it may be a future therapeutic agent in the treatment of complications. Introduction was found to be 81.6%.2 The 2-, 5-, and 10-year metastasis rates were reported to be 10%, 25%, and 34%, respectively, regardless Uveal melanoma is the most common primary intraocular of tumor size.3 tumor in adults. -
Case Report Comment
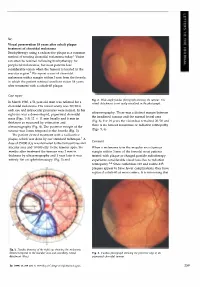
Sir, Visual preservation 18 years after cobalt plaque treatment of choroidal melanoma Brachytherapy using a radioactive plaque is a common method of treating choroidal melanoma today.l Vision can often be retained following brachytherapy for peripheral melanomas, but most patients lose considerable vision when the tumour is located in the macular region.2 We report a case of choroidal melanoma with a margin within 3 mm from the foveola in which the patient retained excellent vision 18 years after treatment with a cobalt-60 plaque. Case report Fig. 2. Wide-angle fundus photograph showing the tumour. The In March 1980, a 51-year-old man was referred for a retinal detachment is not easily visualised in the photograph. choroidal melanoma. His visual acuity was 20/20 in each eye and intraocular pressures were normal. In his ultrasonography. There was a distinct margin between right eye was a dome-shaped, pigmented choroidal the irradiated tumour and the normal foveal area mass (Figs. 1-3) 12 X 11 mm basally and 6 mm in (Fig. 6). For 18 years the vision has remained 20/20 and thickness as measured by estimation and there is no tumour recurrence or radiation retinopathy ultrasonography (Fig. 4). The posterior margin of the (Figs. 5, 6). tumour was 3 mm temporal to the foveola (Fig. 3). The patient elected treatment with a radioactive plaque, which was done by our standard technique. 1 A Comment dose of 35 000 cGy was delivered to the tumour base and macular area and 10 000 cGy to the tumour apex. Six When a melanoma is in the macular area (tumour months after treatment the tumour was 2 mm in margin within 3 mm of the foveola) most patients thickness by ultrasonography and 1 year later it was treated with plaque or charged particle radiotherapy entirely flat on ophthalmoscopy (Fig. -

Diagnosis, and Treatment of Retinoblastoma
British3'ournal ofOphthalmology 1993; 77: 805-812 805 PERSPECTIVE Br J Ophthalmol: first published as 10.1136/bjo.77.12.805 on 1 December 1993. Downloaded from Applications ofmonoclonal antibodies in the investigation, diagnosis, and treatment of retinoblastoma John F Tarlton, D L Easty In Europe and North America, retinoblastoma has a reported derived cell lines fused with RB1 competent cells.2' Reversal incidence ranging from 1 in 14 000 to 1 in 36 000 live births'-5 of malignancy has also been reported in cell lines transfected and is the most common ocular malignancy affecting children with functional BR1 gene constructs. Recently, however, and infants. Early diagnosis allows the application of a these results have been challenged by other researchers who number of therapies directed at localised disease, including have found incomplete reversal of malignancy in retino- external beam and plaque radiotherapy, cryotherapy, and blastoma cell lines,22 although such effects may be artefacts photocoagulation, although the principal treatment account- resulting from additional genetic changes having occurred in ing for the high cure rate ofaround 90% is enucleation, which cells adapting to tissue culture environments. The impor- is still carried out in over halfofall cases. The justification for tance of RB1 in malignant transformation has also been such radical therapy is the poor prognosis once the tumour demonstrated by inactivating its product by association with escapes the eye, and the high mortality from metastases.6 virus proteins such as adenovirus EIA,23 SV40 large T In the developing countries ofAfrica and Asia the picture is antigen24 and papillomavirus E7 oncoprotein,2" both by different. -

Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy
applied sciences Review Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy Corrado Spatola 1,2, Rocco Luca Emanuele Liardo 2, Roberto Milazzotto 2 , Luigi Raffaele 2, Vincenzo Salamone 2, Antonio Basile 1,2, Pietro Valerio Foti 1,2, Stefano Palmucci 1,2 , Giuseppe Antonio Pablo Cirrone 3, Giacomo Cuttone 3, Andrea Russo 4 , Teresio Avitabile 4, Michele Reibaldi 5 , Antonio Longo 4, Giuseppe Broggi 1 , Vincenza Bonfiglio 6, Rosario Caltabiano 1 , Stefano Pergolizzi 7 and Floriana Arena 7,* 1 Dipartimento di Scienze Mediche, Chirurgiche e Tecnologie Avanzate “G.F. Ingrassia”, Università di Catania, 95125 Catania, Italy; [email protected] (C.S.); [email protected] (A.B.); [email protected] (P.V.F.); [email protected] (S.P.); [email protected] (G.B.); [email protected] (R.C.) 2 UO Radiodiagnostica e Radioterapia Oncologica AOU Policlinico-S.Marco Catania, 95125 Catania, Italy; [email protected] (R.L.E.L.); [email protected] (R.M.); raff[email protected] (L.R.); [email protected] (V.S.) 3 Istituto Nazionale di Fisica Nucleare-LNS Catania, 95125 Catania, Italy; [email protected] (G.A.P.C.); [email protected] (G.C.) 4 Dipartimento di Chirurgia Generale e Specialità Medico-Chirurgiche, Università di Catania, 95125 Catania, Italy; [email protected] (A.R.); [email protected] (T.A.); [email protected] (A.L.) 5 Dipartimento di Oftalmologia, Università di Torino, 10124 Torino, Italy; [email protected] 6 Dipartimento -

Radiation Therapy Coding
Ed 21:01 Radiation March 2021 Missouri Cancer Registry and Research Center Therapy 2021 Show-Me-Tips Coding...Abstracting...Education… Radiation Therapy Coding Radiation Delivery Systems/Approaches and Coding LINEAR ACCELERATOR - Linac If the radiation treatment summary refers to beam energies, such as: • 6X or 6MV, 10X or 10MV • 12X or 12MV, 15X or 15MV Then the treatment modality will always be 02, external beam, photons (a Linac was used to deliver the EBRT treatment) If radiation treatment summary refers to treatment delivery as: • E, eboost, MeV or “en face” Code the treatment modality 04, external beam, electrons (a Linac can also deliver electron therapy) IORT Delivery Technology & Coding IORT FOR BREAST CANCER, Volume: 41 - Partial Breast. Lymph Nodes are not Targeted! Equipment RT Delivery Modality Planning Comments Method Technique Zeiss Intrabeam 50 kVp Linac 02-Photons 02: Low energy x-ray/ Isotope-free. No photon therapy radioactive source used XOFT Axxent 50 kVp Linac 02-Photons 02: Low energy x-ray/ Isotope-free. No photon therapy radioactive source used LIAC 10/12 by Electron 04-Electrons In most cases, Max energy: 10 MeV, Sordina IORT accelerator 04-3D conformal 12 MeV NOVAC by Electron 04-Electrons In most cases, Check with Rad Onc for Sordina IORT accelerator 04-3D conformal planning technique Mobetron Electron 04-Electrons In most cases, Energies: 6, 9, 12 MeV accelerator 04-3D conformal Strut Assisted Ir-192 Sources 09-Brachy, 88 - NA Accelerated partial breast Volume (HDR) Intracavitary HDR irradiation (PBI) Implant -

(12) Patent Application Publication (10) Pub. No.: US 2009/0156881 A1 Stokes (43) Pub
US 20090156881A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0156881 A1 Stokes (43) Pub. Date: Jun. 18, 2009 (54) CONVERGENT WELL IRRADIATING Publication Classification PLAOUE FOR CHOROIDAL MELANOMA (51) Int. Cl. (76) Inventor: John P. Stokes, San Antonio, TX A6M 36/12 (2006.01) (US) A6DF 9/00 (2006.01) (52) U.S. Cl. ............................................................ 6OOf7 Correspondence Address: MEYERTONS, HOOD, KIVLIN, KOWERT & (57) ABSTRACT GOETZEL, P.C. Provided in some embodiments is a device suitable for treat P.O. BOX 398 ing an eye that includes a housing and a plurality offins. The AUSTIN, TX 78767-0398 (US) housing includes a base and a rim coupled to the perimeter of the base. The base and the rim at least partially define a cavity (21) Appl. No.: 12/252,136 in the housing, and the cavity is configured to accept one or more radiation seeds. The plurality of fins at least partially (22) Filed: Oct. 15, 2008 reside within or proximate the cavity of the housing. At least a portion of the fins are configured such that radiation emitted Related U.S. Application Data from one or more radiation seeds positioned in the cavity is (60) Provisional application No. 60/980,079, filed on Oct. substantially directed toward a center portion of the eye dur 15, 2007. ing use. SClera ChOroid Patent Application Publication Jun. 18, 2009 Sheet 1 of 8 US 2009/O156881 A1 C-H > O wis w CN Sa CNwas O C v N. l e CD VN sU O S & U wal w- F S S wis,N ws Patent Application Publication Jun. -

Comparison of Retinoblastoma Reduction for Chemotherapy Vs External Beam Radiotherapy
CLINICAL SCIENCES Comparison of Retinoblastoma Reduction for Chemotherapy vs External Beam Radiotherapy Daniel A. Sussman, MD; Erika Escalona-Benz, MD; Matthew S. Benz, MD; Brandy C. Hayden, BS; William Feuer, MS; Nicole Cicciarelli; Stuart Toledano, MD; Arnold Markoe, MD; Timothy G. Murray, MD Objective: To determine the time course and extent of Results: Twenty-six eyes of 26 patients were evaluated tumor reduction associated with systemic chemo- for tumor response; 18 patients were treated with sys- therapy or external beam radiotherapy (EBRT) in the treat- temic chemotherapy and 8 patients were treated with ment of advanced intraocular retinoblastoma. EBRT. Median follow-up was 36 months. A mean 68% reduction in tumor volume occurred after 1 cycle of che- Methods: Retrospective review of children with Reese- motherapy compared with a 12% reduction at a similar Ellsworth stages IV and V retinoblastoma undergoing pri- time point (1 month) after initiation of EBRT (PϽ.004). mary globe-conserving therapy with either systemic che- There was no statistically significant difference in tu- moreduction or EBRT. Study variables were recorded at mor volume reduction between treatment modalities at baseline, at monthly intervals for the first 6 months, and the 12-month follow-up visit. Both systemic chemore- at 12 months after the initiation of treatment. Tumor vol- duction and EBRT achieved 100% globe conservation and umes were calculated using basal area and height values 100% patient survival in this series. determined by ultrasonography, physical examination, and fundus photographic review. Conclusions: Retinoblastoma reduction exhibits a dif- ferential time course based on the applied primary treat- Main Outcome Measures: Outcome measures in- ment. -

PATIENTS • Guide • Heal
Final Program ASTRO 55TH ANNUAL MEETING Meeting Dates: September 22-25, 2013 • Exhibit Dates: September 22-24, 2013 • Georgia World Congress Center • Atlanta HopePATIENTS • Guide • Heal Download ASTROmobile - The offi cial meeting app. See 17 page for details. ANNUAL MEETING SCIENCE BASED, PATIENT DRIVEN www.astro.org/annualmeeting #ASTRO13 ROOM SERVICE NOW AVAILABLE Medical centers that never considered proton therapy accessible are now installing single-room configurations of the MEVION S250™ system. Thanks to a radically smaller footprint, lower capital outlay, reduced operating costs, and complete system integration, advanced proton therapy is now available at the press of a button. ™ Welcome to the world of High Energy Cancer Care ... for everyone. Visit us at ASTRO 2013 Booth #1253 mevion.com LAD130401 Final Program ASTRO 55TH ANNUAL MEETING Meeting Dates: September 22-25, 2013 • Exhibit Dates: September 22-24, 2013 • Georgia World Congress Center • Atlanta HopePATIENTS • Guide • Heal Download ASTROmobile - The offi cial meeting app. See 17 page for details. HELD IN CONJUNCTION WITH: AMERICAN SOCIETY OF RADIOLOGIC TECHNOLOGISTS (ASRT) ANNUAL MEETING ASSOCIATION OF RESIDENTS IN RADIATION ONCOLOGY (ARRO) SCIENCE BASED, PATIENT DRIVEN SOCIETY FOR RADIATION ONCOLOGY ADMINISTRATORS (SROA) www.astro.org/annualmeeting #ASTRO13 Welcome TO THE 55TH ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY “Patients: Hope, Guide, Heal” Dear Colleagues, On behalf of the American Society for Radiation Oncology, I would like to welcome you to ASTRO’s 55th Annual Meeting. We have developed an exciting program that will provide many opportunities for exchanging scientifi c information as well as professional networking. The theme this year is “Patients: Hope, Guide, Heal.” Given all of the changes we are facing in health care, I feel that it is extremely important to keep our focus on our patients and the best care we can provide them. -

Immunoconjugate Induces Cytotoxicity in Human Uveal Melanoma Cells
In Vitro Targeting of NG2 Antigen by 213Bi-9.2.27 ␣-Immunoconjugate Induces Cytotoxicity in Human Uveal Melanoma Cells Yong Li,1,2 Jian Wang,1,3 Syed M. Abbas Rizvi,1 Martine J. Jager,4 Robert M. Conway,2 Francis A. Billson,2 Barry J. Allen,1,2,3 and Michele C. Madigan2 PURPOSE. To examine uveal melanoma cell lines for the expres- mately 95% of all uveal melanomas. Primary uveal melanomas sion of human melanoma proteoglycan (NG2) using monoclo- may be treated successfully with enucleation, local tumor re- nal antibody (mAb) 9.2.27 and subsequently to assess the in section, laser photocoagulation, plaque radiotherapy, proton vitro specificity and cytotoxicity of mAb 9.2.27 conjugated to beam therapy, or combinations of therapies.1–6 Earlier studies 213 213 the ␣-particle–emitting radioisotope bismuth ( Bi-9.2.27) found minimal benefits using intravenous chemotherapy or for uveal melanoma cells. immunotherapy for primary and metastatic uveal melanoma, METHODS. Immunocytochemistry and flow cytometry were though some success with these modalities has been reported used to examine OCM-1, OCM-3, OCM-8, OMM-1, Mel202 and recently.6–9 Proton beam therapy or local plaque therapy gen- 92–1 melanoma cell lines for NG2 expression. Melanoma cells erally produces good clinical responses.10 However, radiation- were treated with test (213Bi-9.2.27) or control (213Bi-A2) ␣-im- associated morbidity remains a problem and usually involves munoconjugates (AICs). The specific cytotoxicity of 213Bi- long-term visual complications. Most patients with uveal mel- 9.2.27 AIC was evaluated using an MTS (3-(4,5-dimethylthiazol- anoma display no detectable evidence of metastases at the time 2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfenyl)-2H-tetrazolium, of diagnosis; however, within 5 years of enucleation, meta- inner salt) assay. -

Role of Gambogic Acid and Nai131 in A549/DDP Cells
ONCOLOGY LETTERS 13: 37-44, 2017 Role of gambogic acid and NaI131 in A549/DDP cells JING HUANG1, XIAOLI ZHU1, HUAN WANG2, SHUHUA HAN1, LU LIU3, YAN XIE3, DAOZHEN CHEN4, QIANG ZHANG1, LI ZHANG1 and YUE HU1 1Department of Pneumology, Zhongda Hospital, Medical School of Southeast University; 2Department of Clinical Medicine, Medical School of Southeast University; 3Department of Nuclear Medicine, Zhongda Hospital, Southeast University, Nanjing, Jiangsu 210009; 4Department of Central Laboratory, Wuxi Maternity and Child Health Care Hospital, Affiliated to Nanjing Medical University, Wuxi, Jiangsu 210004, P.R. China Received May 14, 2015; Accepted June 17, 2016 DOI: 10.3892/ol.2016.5435 Abstract. Resistance to platinum in tumor tissue is a Introduction considerable barrier against effective lung cancer treatment. Radionuclide therapy is the primary adjuvant treatment, Lung cancer is the leading cause of cancer-associated however, the toxic side effects limit its dosage in the clinical mortality in the USA. In 2015, there was an estimated setting. Therefore, the present study aimed to determine 221,200 novel cases of lung and bronchial cancer diagnosed, whether an NaI131 radiosensitizer could help reduce the toxic and 158,040 mortalities were attributed to lung cancer (1). In side effects of radionuclide therapy. In vitro experiments were China, lung cancer-associated mortality accounts for >20% of conducted to determine whether NaI131 can inhibit platinum all cancer mortalities (2). Currently, chemotherapy regimens resistance in A549/DDP cells, which are cisplatin-resistant containing platinum are the primary therapies for advanced non-small cell lung cancer cells, and whether gambogic acid non‑small cell lung cancer (NSCLC) (3). -

Applications of Nanomaterials for Theranostics of Melanoma
Journal of Nanotheranostics Review Applications of Nanomaterials for Theranostics of Melanoma Guanqiao Jin 1, Pohlee Cheah 2 , Jing Qu 2, Lijuan Liu 1 and Yongfeng Zhao 2,* 1 Department of Radiology, The Affiliated Tumor Hospital of Guangxi Medical University, Hedi Road No.71, Nanning 530021, China; [email protected] (G.J.); [email protected] (L.L.) 2 Department of Chemistry, Physics and Atmospheric Science, Jackson State University, Jackson, MS 39217, USA; [email protected] (P.C.); [email protected] (J.Q.) * Correspondence: [email protected] Academic Editor: Clare Hoskins Received: 8 September 2020; Accepted: 13 October 2020; Published: 16 October 2020 Abstract: Melanoma is an aggressive form of skin cancer with a very high mortality rate. Early diagnosis of the disease, the utilization of more potent pharmacological agents, and more effective drug delivery systems are essential to achieve an optimal treatment plan. The applications of nanotechnology to improve therapeutic efficacy and early diagnosis for melanoma treatment have received great interest among researchers and clinicians. In this review, we summarize the recent progress of utilizing various nanomaterials for theranostics of melanoma. The key importance of using nanomaterials for theranostics of melanoma is to improve efficacy and reduce side effects, ensuring safe implementation in clinical use. As opposed to conventional in vitro diagnostic methods, in vivo medical imaging technologies have the advantages of being a type of non-invasive, real-time monitoring. Several common nanoparticles, including ultrasmall superparamagnetic iron oxide nanoparticles, silica nanoparticles, and carbon-based nanoparticles, have been applied to deliver chemotherapeutic agents for the theranostics of melanoma. -

Charged-Particle (Proton Or Helium Ion) Radiotherapy for Neoplastic Conditions Page 1 of 34
Charged-Particle (Proton or Helium Ion) Radiotherapy for Neoplastic Conditions Page 1 of 34 No review or update is scheduled on this Medical Policy as it is unlikely that further published literature would change the policy position. There were no claims being received for the service at the time of archiving. If there are questions about coverage of this service, please contact Blue Cross and Blue Shield of Kansas customer service, your professional or institutional relations representative, or submit a predetermination request. Medical Policy An Independent licensee of the Blue Cross Blue Shield Association Title: Charged-Particle (Proton or Helium Ion) Radiotherapy for Neoplastic Conditions Professional Institutional Original Effective Date: May 1, 2007 Original Effective Date: October 6, 2011 Revision Date(s): October 6, 2011; Revision Date(s): August 6, 2013; August 6, 2013; December 11, 2013; December 11, 2013; November 12, 2015; November 12, 2015; September 1, 2016; September 1, 2016; April 12, 2017; April 12, 2017; August 15, 2017; August 15, 2017; August 29, 2018 August 29, 2018 Current Effective Date: April 12, 2017 Current Effective Date: April 12, 2017 Archive Date: March 9, 2021 Archive Date: March 9, 2021 State and Federal mandates and health plan member contract language, including specific provisions/exclusions, take precedence over Medical Policy and must be considered first in determining eligibility for coverage. To verify a member's benefits, contact Blue Cross and Blue Shield of Kansas Customer Service. The BCBSKS Medical Policies contained herein are for informational purposes and apply only to members who have health insurance through BCBSKS or who are covered by a self-insured group plan administered by BCBSKS.