Charged-Particle (Proton Or Helium Ion) Radiotherapy for Neoplastic Conditions Page 1 of 34
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -
Slowing Down of a Particle Beam in the Dusty Plasmas with Kappa
arXiv:1708.04525 Slowing Down of Charged Particles in Dusty Plasmas with Power-law Kappa-distributions Jiulin Du 1*, Ran Guo 2, Zhipeng Liu3 and Songtao Du4 1 Department of Physics, School of Science, Tianjin University, Tianjin 300072, China 2 School of Science, Civil Aviation University of China, Tianjin 300300, China 3 School of Science, Tianjin Chengjian University, Tianjin 300384, China 4 College of Electronic Information and Automation, Civil Aviation University of China, Tianjin 300300, China Keywords: Slowing down, Kappa-distributions, Dusty plasma, Fokker-Planck collision theory Abstract We study slowing down of a particle beam passing through the dusty plasma with power-law κ-distributions. Three plasma components, electrons, ions and dust particles, can have a different κ-parameter. The deceleration factor and slowing down time are derived and expressed by a hyper-geometric κ-function. Numerically we study slowing down property of an electron beam in the κ-distributed dusty plasma. We show that the slowing down in the plasma depends strongly on the κ-parameters of plasma components, and dust particles play a dominant role in the deceleration effects. We also show dependence of the slowing down on mass and charge of a dust particle in the plasma. 1 Introduction Dusty plasmas are ubiquitous in astrophysical, space and terrestrial environments, such as the interstellar clouds, the circumstellar clouds, the interplanetary space, the comets, the planetary rings, the Earth’s atmosphere, and the lower ionosphere etc. They can also exist in laboratory plasma environments. Dusty plasma consists of three components: electrons, ions and dust particles of micron- or/and submicron-sized particulates. -

Upgrading the CMS Detector on the Large Hadron Collider
Upgrading the CMS Detector on the Large Hadron Collider Stefan Spanier Professor, Department of Physics CURRENT RESEARCH AFFILIATION Putting a diamond on the largest ring in the world The University of Tennessee, Knoxville July 4, 2012 is one of the most exciting dates to remember in modern science. On this day, EDUCATION scientists working with the Large Hadron Collider (LHC) at the European Organization for Nuclear Research (CERN) in Switzerland discovered a particle consistent with the Ph.D., Johannes Gutenberg University, Mainz, Germany characteristics of the Higgs boson particle. This particle is predicted by the Standard Model of particle physics. The model is very successful in linking measurements made with previous RESEARCH AREAS particle accelerators, but still leaves unanswered questions about how the universe works. The model does ignores dark matter and dark energy, and while it describes the behavior of Technology, Materials Science / Physics the Higgs particle it does not predict its own mass. These mysteries indicate there must be a larger picture that includes new forces and particles, and the Standard Model is only part of FUNDING REQUEST it. Spanier is seeking $70,000 annually to fund this project. This will cover a graduate student This is where the LHC, the world’s largest and most powerful particle accelerator comes in. for one year, travel expenses to the particle beam physics laboratory, acquisition and The LHC was first conceived in 1984, and brought online in 2008. It consists of a 27-kilometer preparation of a new detector substrate from a diamond growth process batch and one ring of superconducting magnets with accelerating structures that boost protons to neutron irradiation in a nuclear reactor. -

Decays of the Tau Lepton*
SLAC - 292 UC - 34D (E) DECAYS OF THE TAU LEPTON* Patricia R. Burchat Stanford Linear Accelerator Center Stanford University Stanford, California 94305 February 1986 Prepared for the Department of Energy under contract number DE-AC03-76SF00515 Printed in the United States of America. Available from the National Techni- cal Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, Virginia 22161. Price: Printed Copy A07, Microfiche AOl. JC Ph.D. Dissertation. Abstract Previous measurements of the branching fractions of the tau lepton result in a discrepancy between the inclusive branching fraction and the sum of the exclusive branching fractions to final states containing one charged particle. The sum of the exclusive branching fractions is significantly smaller than the inclusive branching fraction. In this analysis, the branching fractions for all the major decay modes are measured simultaneously with the sum of the branching fractions constrained to be one. The branching fractions are measured using an unbiased sample of tau decays, with little background, selected from 207 pb-l of data accumulated with the Mark II detector at the PEP e+e- storage ring. The sample is selected using the decay products of one member of the r+~- pair produced in e+e- annihilation to identify the event and then including the opposite member of the pair in the sample. The sample is divided into subgroups according to charged and neutral particle multiplicity, and charged particle identification. The branching fractions are simultaneously measured using an unfold technique and a maximum likelihood fit. The results of this analysis indicate that the discrepancy found in previous experiments is possibly due to two sources. -

The Role of Combined Therapy in the Treatment of Retinopathy and Optic Neuropathy Due to Radiotherapy in the Uveal Melanoma
Published online: 2020-01-16 THIEME Original Article 1 The Role of Combined Therapy in the Treatment of Retinopathy and Optic Neuropathy Due to Radiotherapy in the Uveal Melanoma Yasemin Benderli Cihan1 1Department of Radiation Oncology, Kayseri Education and Address for correspondence Yasemin Benderli Cihan, MD, Department Research Hospital, Kayseri, Turkey of Radiation Oncology, Kayseri Education and Research Hospital, Sanayi District, Ataturk Boulevard, Hastane Street, No 78, 38010 Kocasinan, Kayseri, Turkey (e-mail: [email protected]). Asian J Oncol 2020;6:1–2 Abstract Introduction Uveal melanoma has a relatively low incidence. Transpupillary thermo- therapy (TTT), hypofractioned stereotactic radiotherapy (RT), stereotactic radiosur- gery, plaque brachytherapy, charged particle radiation therapy, local tumor resection, enucleation, and exantation are applied in the treatment. Methods The importance given to radiotherapy has increased to get more satisfac- tory results while treating the patient. However, it is the treatment of radiation reti- nopathy and optic neuropathy from complications. Results Radiation retinopathy and optic neuropathy are the most important compli- Keywords cations related to radiotherapy in the treatment of uveal melanoma. In recent years, ► uveal melanoma many studies have been performed on the treatment of radiation retinopathy and ► radiotherapy optic neuropathy. ► retinopathy Conclusion The consecutive use of triamcinolone in combination with anti-VEGF ► optic neuropathy supports that it may be a future therapeutic agent in the treatment of complications. Introduction was found to be 81.6%.2 The 2-, 5-, and 10-year metastasis rates were reported to be 10%, 25%, and 34%, respectively, regardless Uveal melanoma is the most common primary intraocular of tumor size.3 tumor in adults. -

Particle Beam Diagnostics and Control
Particle beam diagnostics and control G. Kube Deutsches Elektronen-Synchrotron DESY Notkestraße 85, 22607 Hamburg, Germany Summary. | Beam diagnostics and instrumentation are an essential part of any kind of accelerator. There is a large variety of parameters to be measured for obser- vation of particle beams with the precision required to tune, operate and improve the machine. Depending on the type of accelerator, for the same parameter the working principle of a monitor may strongly differ, and related to it also the requirements for accuracy. This report will mainly focus on electron beam diagnostic monitors presently in use at 4th generation light sources (single-pass Free Electron Lasers), and present the state-of-the-art diagnostic systems and concepts. 1. { Introduction Nowadays particle accelerators play an important role in a wide number of fields where a primary or secondary beam from an accelerator can be used for industrial or medical applications or for basic and applied research. The interaction of such beam with matter is exploited in order to analyze physical, chemical or biological samples, for a modification of physical, chemical or biological sample properties, or for fundamental research in basic subatomic physics. In order to cover such a wide range of applications different accelerator types are required. Cyclotrons are often used to produce medical isotopes for positron emission to- ⃝c Societ`aItaliana di Fisica 1 2 G. Kube mography (PET) and single photon emission computed tomography (SPECT). For elec- tron radiotherapy mainly linear accelerators (linacs) are in operation, while cyclotrons or synchrotrons are additionally used for proton therapy. Third generation synchrotron light sources are electron synchrotrons, while the new fourth generation light sources (free electron lasers) operating at short wavelengths are electron linac based accelera- tors. -

4. Particle Generators/Accelerators
Joint innovative training and teaching/ learning program in enhancing development and transfer knowledge of application of ionizing radiation in materials processing 4. Particle Generators/Accelerators Diana Adlienė Department of Physics Kaunas University of Technolog y Joint innovative training and teaching/ learning program in enhancing development and transfer knowledge of application of ionizing radiation in materials processing This project has been funded with support from the European Commission. This publication reflects the views only of the author. Polish National Agency and the Commission cannot be held responsible for any use which may be made of the information contained therein. Date: Oct. 2017 DISCLAIMER This presentation contains some information addapted from open access education and training materials provided by IAEA TABLE OF CONTENTS 1. Introduction 2. X-ray machines 3. Particle generators/accelerators 4. Types of industrial irradiators The best accelerator in the universe… INTRODUCTION • Naturally occurring radioactive sources: – Up to 5 MeV Alpha’s (helium nuclei) – Up to 3 MeV Beta particles (electrons) • Natural sources are difficult to maintain, their applications are limited: – Chemical processing: purity, messy, and expensive; – Low intensity; – Poor geometry; – Uncontrolled energies, usually very broad Artificial sources (beams) are requested! INTRODUCTION • Beams of accelerated particles can be used to produce beams of secondary particles: Photons (x-rays, gamma-rays, visible light) are generated from beams -

Beam-Transport Systems for Particle Therapy
Beam-Transport Systems for Particle Therapy J.M. Schippers Paul Scherrer Institut, Villigen, Switzerland Abstract The beam transport system between accelerator and patient treatment location in a particle therapy facility is described. After some general layout aspects the major beam handling tasks of this system are discussed. These are energy selection, an optimal transport of the particle beam to the beam delivery device and the gantry, a device that is able to rotate a beam delivery system around the patient, so that the tumour can be irradiated from almost any direction. Also the method of pencil beam scanning is described and how this is implemented within a gantry. Using this method the particle dose is spread over the tumour volume to the prescribed dose distribution. Keywords Beam transport; beam optics; degrader; beam analysis; gantry; pencil beam scanning. 1 Introduction The main purpose of the beam-transport system is to aim the proton beam, with the correct diameter and intensity, at the tumour in the patient and to apply the correct dose distribution. The beam transport from the accelerator to the tumour in the patient consists of the following major sections (see Fig. 1): – energy setting and energy selection (only for cyclotrons); – transport system to the treatment room(s), including beam-emittance matching; – per treatment room—a gantry or a fixed beam line aiming the beam from the correct direction; – beam-delivery system in the treatment room, by which the dose distribution is actually being applied. These devices are combined in the so called ‘nozzle’ at the exit of the fixed beam line or of the gantry. -

Charged Particle Radiotherapy
Corporate Medical Policy Charged Particle Radiotherapy File Name: charged_particle_radiotherapy Origination: 3/12/96 Last CAP Review: 5/2021 Next CAP Review: 5/2022 Last Review: 5/2021 Description of Procedure or Service Cha rged-particle beams consisting of protons or helium ions or carbon ions are a type of particulate ra dia tion therapy (RT). They contrast with conventional electromagnetic (i.e., photon) ra diation therapy due to several unique properties including minimal scatter as particulate beams pass through tissue, and deposition of ionizing energy at precise depths (i.e., the Bragg peak). Thus, radiation exposure of surrounding normal tissues is minimized. The theoretical advantages of protons and other charged-particle beams may improve outcomes when the following conditions a pply: • Conventional treatment modalities do not provide adequate local tumor control; • Evidence shows that local tumor response depends on the dose of radiation delivered; and • Delivery of adequate radiation doses to the tumor is limited by the proximity of vital ra diosensitive tissues or structures. The use of proton or helium ion radiation therapy has been investigated in two general categories of tumors/abnormalities. However, advances in photon-based radiation therapy (RT) such as 3-D conformal RT, intensity-modulated RT (IMRT), a nd stereotactic body ra diotherapy (SBRT) a llow improved targeting of conventional therapy. 1. Tumors located near vital structures, such as intracranial lesions or lesions a long the a xial skeleton, such that complete surgical excision or adequate doses of conventional radiation therapy are impossible. These tumors/lesions include uveal melanomas, chordomas, and chondrosarcomas at the base of the skull and a long the axial skeleton. -

Beam–Material Interactions
Beam–Material Interactions N.V. Mokhov1 and F. Cerutti2 1Fermilab, Batavia, IL 60510, USA 2CERN, Geneva, Switzerland Abstract This paper is motivated by the growing importance of better understanding of the phenomena and consequences of high-intensity energetic particle beam interactions with accelerator, generic target, and detector components. It reviews the principal physical processes of fast-particle interactions with matter, effects in materials under irradiation, materials response, related to component lifetime and performance, simulation techniques, and methods of mitigating the impact of radiation on the components and environment in challenging current and future applications. Keywords Particle physics simulation; material irradiation effects; accelerator design. 1 Introduction The next generation of medium- and high-energy accelerators for megawatt proton, electron, and heavy- ion beams moves us into a completely new domain of extreme energy deposition density up to 0.1 MJ/g and power density up to 1 TW/g in beam interactions with matter [1, 2]. The consequences of controlled and uncontrolled impacts of such high-intensity beams on components of accelerators, beamlines, target stations, beam collimators and absorbers, detectors, shielding, and the environment can range from minor to catastrophic. Challenges also arise from the increasing complexity of accelerators and experimental set-ups, as well as from design, engineering, and performance constraints. All these factors put unprecedented requirements on the accuracy of particle production predictions, the capability and reliability of the codes used in planning new accelerator facilities and experiments, the design of machine, target, and collimation systems, new materials and technologies, detectors, and radiation shielding and the minimization of radiation impact on the environment. -
Case Report Comment
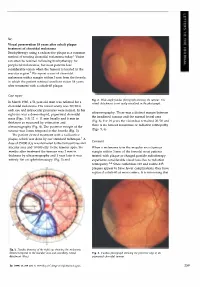
Sir, Visual preservation 18 years after cobalt plaque treatment of choroidal melanoma Brachytherapy using a radioactive plaque is a common method of treating choroidal melanoma today.l Vision can often be retained following brachytherapy for peripheral melanomas, but most patients lose considerable vision when the tumour is located in the macular region.2 We report a case of choroidal melanoma with a margin within 3 mm from the foveola in which the patient retained excellent vision 18 years after treatment with a cobalt-60 plaque. Case report Fig. 2. Wide-angle fundus photograph showing the tumour. The In March 1980, a 51-year-old man was referred for a retinal detachment is not easily visualised in the photograph. choroidal melanoma. His visual acuity was 20/20 in each eye and intraocular pressures were normal. In his ultrasonography. There was a distinct margin between right eye was a dome-shaped, pigmented choroidal the irradiated tumour and the normal foveal area mass (Figs. 1-3) 12 X 11 mm basally and 6 mm in (Fig. 6). For 18 years the vision has remained 20/20 and thickness as measured by estimation and there is no tumour recurrence or radiation retinopathy ultrasonography (Fig. 4). The posterior margin of the (Figs. 5, 6). tumour was 3 mm temporal to the foveola (Fig. 3). The patient elected treatment with a radioactive plaque, which was done by our standard technique. 1 A Comment dose of 35 000 cGy was delivered to the tumour base and macular area and 10 000 cGy to the tumour apex. Six When a melanoma is in the macular area (tumour months after treatment the tumour was 2 mm in margin within 3 mm of the foveola) most patients thickness by ultrasonography and 1 year later it was treated with plaque or charged particle radiotherapy entirely flat on ophthalmoscopy (Fig. -

The Basic Interactions Between Photons and Charged Particles With
Outline Chapter 6 The Basic Interactions between • Photon interactions Photons and Charged Particles – Photoelectric effect – Compton scattering with Matter – Pair productions Radiation Dosimetry I – Coherent scattering • Charged particle interactions – Stopping power and range Text: H.E Johns and J.R. Cunningham, The – Bremsstrahlung interaction th physics of radiology, 4 ed. – Bragg peak http://www.utoledo.edu/med/depts/radther Photon interactions Photoelectric effect • Collision between a photon and an • With energy deposition atom results in ejection of a bound – Photoelectric effect electron – Compton scattering • The photon disappears and is replaced by an electron ejected from the atom • No energy deposition in classical Thomson treatment with kinetic energy KE = hν − Eb – Pair production (above the threshold of 1.02 MeV) • Highest probability if the photon – Photo-nuclear interactions for higher energies energy is just above the binding energy (above 10 MeV) of the electron (absorption edge) • Additional energy may be deposited • Without energy deposition locally by Auger electrons and/or – Coherent scattering Photoelectric mass attenuation coefficients fluorescence photons of lead and soft tissue as a function of photon energy. K and L-absorption edges are shown for lead Thomson scattering Photoelectric effect (classical treatment) • Electron tends to be ejected • Elastic scattering of photon (EM wave) on free electron o at 90 for low energy • Electron is accelerated by EM wave and radiates a wave photons, and approaching • No