Applications of Nanomaterials for Theranostics of Melanoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Combined Therapy in the Treatment of Retinopathy and Optic Neuropathy Due to Radiotherapy in the Uveal Melanoma
Published online: 2020-01-16 THIEME Original Article 1 The Role of Combined Therapy in the Treatment of Retinopathy and Optic Neuropathy Due to Radiotherapy in the Uveal Melanoma Yasemin Benderli Cihan1 1Department of Radiation Oncology, Kayseri Education and Address for correspondence Yasemin Benderli Cihan, MD, Department Research Hospital, Kayseri, Turkey of Radiation Oncology, Kayseri Education and Research Hospital, Sanayi District, Ataturk Boulevard, Hastane Street, No 78, 38010 Kocasinan, Kayseri, Turkey (e-mail: [email protected]). Asian J Oncol 2020;6:1–2 Abstract Introduction Uveal melanoma has a relatively low incidence. Transpupillary thermo- therapy (TTT), hypofractioned stereotactic radiotherapy (RT), stereotactic radiosur- gery, plaque brachytherapy, charged particle radiation therapy, local tumor resection, enucleation, and exantation are applied in the treatment. Methods The importance given to radiotherapy has increased to get more satisfac- tory results while treating the patient. However, it is the treatment of radiation reti- nopathy and optic neuropathy from complications. Results Radiation retinopathy and optic neuropathy are the most important compli- Keywords cations related to radiotherapy in the treatment of uveal melanoma. In recent years, ► uveal melanoma many studies have been performed on the treatment of radiation retinopathy and ► radiotherapy optic neuropathy. ► retinopathy Conclusion The consecutive use of triamcinolone in combination with anti-VEGF ► optic neuropathy supports that it may be a future therapeutic agent in the treatment of complications. Introduction was found to be 81.6%.2 The 2-, 5-, and 10-year metastasis rates were reported to be 10%, 25%, and 34%, respectively, regardless Uveal melanoma is the most common primary intraocular of tumor size.3 tumor in adults. -

ASTRO Bone Metastases Guideline-Full Version
1 Palliative Radiotherapy for Bone Metastases: An ASTRO Evidence-Based Guideline Stephen T. Lutz, M.D.,* Lawrence B. Berk, M.D., Ph.D.,† Eric L. Chang, M.D.,‡ Edward Chow, M.B.B.S.,§ Carol A. Hahn, M.D.,║ Peter J. Hoskin, M.D.,¶ David D. Howell, M.D.,# Andre A. Konski, M.D.,** Lisa A. Kachnic, M.D.,†† Simon S. Lo, M.B. ChB,§§ Arjun Sahgal, M.D.,║║ Larry N. Silverman, M.D.,¶¶ Charles von Gunten, M.D., Ph.D., FACP,## Ehud Mendel, M.D., FACS,*** Andrew D. Vassil, M.D.,††† Deborah Watkins Bruner, R.N., Ph.D.,‡‡‡ and William F. Hartsell, M.D.§§§ * Department of Radiation Oncology, Blanchard Valley Regional Cancer Center, Findlay, Ohio; † Department of Radiation Oncology, Moffitt Cancer Center, Tampa, Florida; ‡ Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas; § Department of Radiation Oncology, Sunnybrook Odette Cancer Center, University of Toronto, Toronto, Ontario, Canada; ║ Department of Radiation Oncology, Duke University, Durham, North Carolina; ¶ Mount Vernon Centre for Cancer Treatment, Middlesex, UK; # Department of Radiation Oncology, University of Michigan, Mt. Pleasant, Michigan; ** Department of Radiation Oncology, Wayne State University, Detroit, Michigan; †† Department of Radiation Oncology, Boston Medical Center, Boston, Massachusetts; §§ Department of Radiation Oncology, Ohio State University, Columbus, Ohio; ║║ Department of Radiation Oncology, Sunnybrook Odette Cancer Center and the Princess Margaret Hospital, University of Toronto, Toronto, Ontario, Canada; ¶¶ 21st Century Oncology, Sarasota, Florida; ## The Institute for Palliative Medicine, San Diego Hospice, San Diego, California; *** Neurological Surgery, Ohio State University, Columbus, Ohio; ††† Department of Radiation Oncology, The Cleveland Clinic 2 Foundation, Cleveland, Ohio; ‡‡‡ School of Nursing, University of Pennsylvania, Philadelphia, Pennsylvania; §§§ Department of Radiation Oncology, Good Samaritan Cancer Center, Downers Grove, Illinois Reprint requests to: Stephen Lutz, M.D., 15990 Medical Drive South, Findlay, OH 45840. -

Cancer Treatment and Survivorship Facts & Figures 2019-2021
Cancer Treatment & Survivorship Facts & Figures 2019-2021 Estimated Numbers of Cancer Survivors by State as of January 1, 2019 WA 386,540 NH MT VT 84,080 ME ND 95,540 59,970 38,430 34,360 OR MN 213,620 300,980 MA ID 434,230 77,860 SD WI NY 42,810 313,370 1,105,550 WY MI 33,310 RI 570,760 67,900 IA PA NE CT 243,410 NV 185,720 771,120 108,500 OH 132,950 NJ 543,190 UT IL IN 581,350 115,840 651,810 296,940 DE 55,460 CA CO WV 225,470 1,888,480 KS 117,070 VA MO MD 275,420 151,950 408,060 300,200 KY 254,780 DC 18,750 NC TN 470,120 AZ OK 326,530 NM 207,260 AR 392,530 111,620 SC 143,320 280,890 GA AL MS 446,900 135,260 244,320 TX 1,140,170 LA 232,100 AK 36,550 FL 1,482,090 US 16,920,370 HI 84,960 States estimates do not sum to US total due to rounding. Source: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. Contents Introduction 1 Long-term Survivorship 24 Who Are Cancer Survivors? 1 Quality of Life 24 How Many People Have a History of Cancer? 2 Financial Hardship among Cancer Survivors 26 Cancer Treatment and Common Side Effects 4 Regaining and Improving Health through Healthy Behaviors 26 Cancer Survival and Access to Care 5 Concerns of Caregivers and Families 28 Selected Cancers 6 The Future of Cancer Survivorship in Breast (Female) 6 the United States 28 Cancers in Children and Adolescents 9 The American Cancer Society 30 Colon and Rectum 10 How the American Cancer Society Saves Lives 30 Leukemia and Lymphoma 12 Research 34 Lung and Bronchus 15 Advocacy 34 Melanoma of the Skin 16 Prostate 16 Sources of Statistics 36 Testis 17 References 37 Thyroid 19 Acknowledgments 45 Urinary Bladder 19 Uterine Corpus 21 Navigating the Cancer Experience: Treatment and Supportive Care 22 Making Decisions about Cancer Care 22 Cancer Rehabilitation 22 Psychosocial Care 23 Palliative Care 23 Transitioning to Long-term Survivorship 23 This publication attempts to summarize current scientific information about Global Headquarters: American Cancer Society Inc. -
Case Report Comment
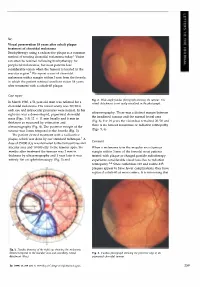
Sir, Visual preservation 18 years after cobalt plaque treatment of choroidal melanoma Brachytherapy using a radioactive plaque is a common method of treating choroidal melanoma today.l Vision can often be retained following brachytherapy for peripheral melanomas, but most patients lose considerable vision when the tumour is located in the macular region.2 We report a case of choroidal melanoma with a margin within 3 mm from the foveola in which the patient retained excellent vision 18 years after treatment with a cobalt-60 plaque. Case report Fig. 2. Wide-angle fundus photograph showing the tumour. The In March 1980, a 51-year-old man was referred for a retinal detachment is not easily visualised in the photograph. choroidal melanoma. His visual acuity was 20/20 in each eye and intraocular pressures were normal. In his ultrasonography. There was a distinct margin between right eye was a dome-shaped, pigmented choroidal the irradiated tumour and the normal foveal area mass (Figs. 1-3) 12 X 11 mm basally and 6 mm in (Fig. 6). For 18 years the vision has remained 20/20 and thickness as measured by estimation and there is no tumour recurrence or radiation retinopathy ultrasonography (Fig. 4). The posterior margin of the (Figs. 5, 6). tumour was 3 mm temporal to the foveola (Fig. 3). The patient elected treatment with a radioactive plaque, which was done by our standard technique. 1 A Comment dose of 35 000 cGy was delivered to the tumour base and macular area and 10 000 cGy to the tumour apex. Six When a melanoma is in the macular area (tumour months after treatment the tumour was 2 mm in margin within 3 mm of the foveola) most patients thickness by ultrasonography and 1 year later it was treated with plaque or charged particle radiotherapy entirely flat on ophthalmoscopy (Fig. -

Predictors of Survival in Acute Myeloid Leukemia by Treatment Modality
ANTICANCER RESEARCH 36: 1719-1728 (2016) Predictors of Survival in Acute Myeloid Leukemia by Treatment Modality SAMIP MASTER1, RICHARD MANSOUR1, SRINIVAS S. DEVARAKONDA1, ZHENZHEN SHI2, GLENN MILLS1 and RUNHUA SHI1 1Department of Medicine & Feist-Weiller Cancer Center, Louisiana State University Health Shreveport, Shreveport, LA, U.S.A.; 2Weill Cornell Medical College, New York, NY, U.S.A. Abstract. Background/Aim: Evaluations of efficacy of distance less than 30 miles from treatment Center, diagnosis treatment modality in analyses on patients with acute myeloid and treatment at same facility, were independently associated leukemia (AML) often combine chemotherapy and stem cell with worse survival. Conclusion: Survival analysis of AML in transplantation (SCT). To account for the effect of SCT and the National Cancer Database showed multiple factors to be determine the impact of chemotherapy alone, the National independently associated with survival. Outcomes based on Cancer Data Base from 1998-2011 was analyzed. Patients and treatment suggest an improved survival when utilizing Methods: Patients with AML from 1998-2011 aged 18-64 years chemotherapy and SCT as the primary treatment modality. were included. Chi-square analysis was used to assess the association between treatment and factors investigated. The The American Cancer Society estimated there were Kaplan–Meier method was used to assess overall survival. approximately 20,830 new cases and 10,460 deaths from Log-rank methods were used to determine factors significant acute myeloid leukemia (AML) in 2015 (1). AML is for survival. Multivariable Cox regression analysis was used to generally a disease of older people and uncommon before determine the effect of chemotherapy alone, and both the age of 45 years. -

Nanocarriers As Magic Bullets in the Treatment of Leukemia
nanomaterials Review Nanocarriers as Magic Bullets in the Treatment of Leukemia 1, 2, 1 2 Mohammad Houshmand y , Francesca Garello y , Paola Circosta , Rachele Stefania , Silvio Aime 2, Giuseppe Saglio 1 and Claudia Giachino 1,* 1 Department of Clinical and Biological Sciences, University of Torino, 10043 Torino, Italy; [email protected] (M.H.); [email protected] (P.C.); [email protected] (G.S.) 2 Molecular and Preclinical Imaging Centres, Department of Molecular Biotechnology and Health Sciences, University of Torino, 10126 Torino, Italy; [email protected] (F.G.); [email protected] (R.S.); [email protected] (S.A.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 23 December 2019; Accepted: 1 February 2020; Published: 6 February 2020 Abstract: Leukemia is a type of hematopoietic stem/progenitor cell malignancy characterized by the accumulation of immature cells in the blood and bone marrow. Treatment strategies mainly rely on the administration of chemotherapeutic agents, which, unfortunately, are known for their high toxicity and side effects. The concept of targeted therapy as magic bullet was introduced by Paul Erlich about 100 years ago, to inspire new therapies able to tackle the disadvantages of chemotherapeutic agents. Currently, nanoparticles are considered viable options in the treatment of different types of cancer, including leukemia. The main advantages associated with the use of these nanocarriers summarized as follows: i) they may be designed to target leukemic cells selectively; ii) they invariably enhance bioavailability and blood circulation half-life; iii) their mode of action is expected to reduce side effects. -

Current Trends in Cancer Immunotherapy
biomedicines Review Current Trends in Cancer Immunotherapy Ivan Y. Filin 1 , Valeriya V. Solovyeva 1 , Kristina V. Kitaeva 1, Catrin S. Rutland 2 and Albert A. Rizvanov 1,3,* 1 Institute of Fundamental Medicine and Biology, Kazan Federal University, 420008 Kazan, Russia; [email protected] (I.Y.F.); [email protected] (V.V.S.); [email protected] (K.V.K.) 2 Faculty of Medicine and Health Science, University of Nottingham, Nottingham NG7 2QL, UK; [email protected] 3 Republic Clinical Hospital, 420064 Kazan, Russia * Correspondence: [email protected]; Tel.: +7-905-316-7599 Received: 9 November 2020; Accepted: 16 December 2020; Published: 17 December 2020 Abstract: The search for an effective drug to treat oncological diseases, which have become the main scourge of mankind, has generated a lot of methods for studying this affliction. It has also become a serious challenge for scientists and clinicians who have needed to invent new ways of overcoming the problems encountered during treatments, and have also made important discoveries pertaining to fundamental issues relating to the emergence and development of malignant neoplasms. Understanding the basics of the human immune system interactions with tumor cells has enabled new cancer immunotherapy strategies. The initial successes observed in immunotherapy led to new methods of treating cancer and attracted the attention of the scientific and clinical communities due to the prospects of these methods. Nevertheless, there are still many problems that prevent immunotherapy from calling itself an effective drug in the fight against malignant neoplasms. This review examines the current state of affairs for each immunotherapy method, the effectiveness of the strategies under study, as well as possible ways to overcome the problems that have arisen and increase their therapeutic potentials. -

Therapies for Clinically Localized Prostate Cancer: Final Report
Comparative Effectiveness Review Number 230 R Therapies for Clinically Localized Prostate Cancer Comparative Effectiveness Review Number 230 Therapies for Clinically Localized Prostate Cancer Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. 290-2015-0000-81 Prepared by: Minnesota Evidence-based Practice Center Minneapolis, MN Investigators: Philipp Dahm, M.D., M.H.S.C. Michelle Brasure, Ph.D., M.S.P.H., M.L.I.S. Elizabeth Ester, M.D. Eric J. Linskens, B.S. Roderick MacDonald, M.S. Victoria A. Nelson, M.Sc. Charles Ryan, M.D. Jayati Saha, Ph.D. Shahnaz Sultan, M.D., M.H.S.C. Kristen E. Ullman, M.P.H. Timothy J. Wilt, M.D., M.P.H. AHRQ Publication No. 20-EHC022 September 2020 This report is based on research conducted by the Minnesota Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2015-0000-81). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. None of the investigators have any affiliations or financial involvement that conflicts with the material presented in this report. The information in this report is intended to help healthcare decision makers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of healthcare services. -

Chemotherapy Treatment and Strategy Schemes: a Review
Review Article Open Acc J of Toxicol Volume 2 Issue 5 - March 2018 Copyright © All rights are reserved by Afroze Alam DOI : 10.19080/OAJT.2018.02.555600 Chemotherapy Treatment and Strategy Schemes: A Review Afroze Alam1,5*, U Farooq2, Ruchi Singh1, VP Dubey1, Shailendra Kumar3, Rashmi Kumari1, Kamlesh Kumar Naik4, BD Tripathi1 and KL Dhar5 1Narayan Institute of Pharmacy, India 2Faculty of Dentistry, Taif University, Saudi Arabia 3Govt Pharmacy Institute, India 4Nandha College of Pharmacy, India 5Faculty of Pharmaceutical Sciences, Shoolini University, India Submission: November 03, 2017; Published: March 29, 2018 *Corresponding author: Afroze Alam, Narayan Institute of Pharmacy, Bihar, India, Tel: ; Fax: +91-1792308000; Email: Abstract Chemotherapy treatments are used to inhibit vigorously growing malignant cells with anticancer agents. This type of therapy may be used to cure and try a patient to produce palliative relief and symptomatic relaxation in the advance stages of cancer. In general, chemotherapy is given in cyclic manners. Patients are administered with anticancer drug during regular weekly or bi-weekly sessions. After the completion of effects of anticancer drugs. Generally, six or more chemotherapeutic cycles are needed in cancer patient and often a combined chemotherapy certain cyclic session, treatment is stopped for several fixed periods to allow the patient to rest and their body to re-establish from the toxic normal circumstances, such as those in the digestive tract, bone marrow and hair follicles. Combination chemotherapy is always preferred overwould traditional be preferred. therapies, Anticancer such drugs as radiotherapy, are used to destroysurgery not or onlyother to cytotoxic specific cancer drugs. cell The but mechanism equally affect of action the normal of each cell therapy that developing is different under for reviewinhibiting describes cell division the alternative and proliferation methods of that rapidly combine growing chemotherapy cell. -

Diagnosis, and Treatment of Retinoblastoma
British3'ournal ofOphthalmology 1993; 77: 805-812 805 PERSPECTIVE Br J Ophthalmol: first published as 10.1136/bjo.77.12.805 on 1 December 1993. Downloaded from Applications ofmonoclonal antibodies in the investigation, diagnosis, and treatment of retinoblastoma John F Tarlton, D L Easty In Europe and North America, retinoblastoma has a reported derived cell lines fused with RB1 competent cells.2' Reversal incidence ranging from 1 in 14 000 to 1 in 36 000 live births'-5 of malignancy has also been reported in cell lines transfected and is the most common ocular malignancy affecting children with functional BR1 gene constructs. Recently, however, and infants. Early diagnosis allows the application of a these results have been challenged by other researchers who number of therapies directed at localised disease, including have found incomplete reversal of malignancy in retino- external beam and plaque radiotherapy, cryotherapy, and blastoma cell lines,22 although such effects may be artefacts photocoagulation, although the principal treatment account- resulting from additional genetic changes having occurred in ing for the high cure rate ofaround 90% is enucleation, which cells adapting to tissue culture environments. The impor- is still carried out in over halfofall cases. The justification for tance of RB1 in malignant transformation has also been such radical therapy is the poor prognosis once the tumour demonstrated by inactivating its product by association with escapes the eye, and the high mortality from metastases.6 virus proteins such as adenovirus EIA,23 SV40 large T In the developing countries ofAfrica and Asia the picture is antigen24 and papillomavirus E7 oncoprotein,2" both by different. -

New First Line Treatment Options of Clear Cell Renal Cell Cancer Patients with PD-1 Or PD-L1 Immune-Checkpoint Inhibitor-Based Combination Therapies
Journal of Clinical Medicine Review New First Line Treatment Options of Clear Cell Renal Cell Cancer Patients with PD-1 or PD-L1 Immune-Checkpoint Inhibitor-Based Combination Therapies Marc-Oliver Grimm 1,*, Katharina Leucht 1, Viktor Grünwald 2 and Susan Foller 1 1 Department of Urology, University Hospital Jena, 07747 Jena, Germany; [email protected] (K.L.); [email protected] (S.F.) 2 Interdisciplinary Urology, Western German Tumor Center Essen, Department for Internal Medicine and Urology, University Hospital Essen, 45127 Essen, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-3641-9329901 Received: 24 January 2020; Accepted: 12 February 2020; Published: 19 February 2020 Abstract: In metastatic renal cell carcinoma (mRCC) the PD-1 immune-checkpoint inhibitor (ICI) Nivolumab became a standard second line treatment option in 2015 based on a significant improvement of overall survival compared to Everolimus. Current pivotal phase 3 studies showed that PD-1 ICI-based combinations were more efficacious than the VEGFR-TKI Sunitinib, a previous standard of care, leading to approval of three new regimens as guideline-recommended first-line treatment. Nivolumab plus Ipilimumab is characterized by a survival advantage, a high rate of complete response and durable remissions in intermediate and poor prognosis patients. Despite frequent immune-mediated side effects, fewer symptoms and a better quality of life were observed compared to Sunitinib. Pembrolizumab or Avelumab plus Axitinib were characterized by an improved progression-free-survival and a high response rate with a low rate of intrinsic resistance. In addition, Pembrolizumab plus Axitinib reached a significant survival benefit. -

Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy
applied sciences Review Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy Corrado Spatola 1,2, Rocco Luca Emanuele Liardo 2, Roberto Milazzotto 2 , Luigi Raffaele 2, Vincenzo Salamone 2, Antonio Basile 1,2, Pietro Valerio Foti 1,2, Stefano Palmucci 1,2 , Giuseppe Antonio Pablo Cirrone 3, Giacomo Cuttone 3, Andrea Russo 4 , Teresio Avitabile 4, Michele Reibaldi 5 , Antonio Longo 4, Giuseppe Broggi 1 , Vincenza Bonfiglio 6, Rosario Caltabiano 1 , Stefano Pergolizzi 7 and Floriana Arena 7,* 1 Dipartimento di Scienze Mediche, Chirurgiche e Tecnologie Avanzate “G.F. Ingrassia”, Università di Catania, 95125 Catania, Italy; [email protected] (C.S.); [email protected] (A.B.); [email protected] (P.V.F.); [email protected] (S.P.); [email protected] (G.B.); [email protected] (R.C.) 2 UO Radiodiagnostica e Radioterapia Oncologica AOU Policlinico-S.Marco Catania, 95125 Catania, Italy; [email protected] (R.L.E.L.); [email protected] (R.M.); raff[email protected] (L.R.); [email protected] (V.S.) 3 Istituto Nazionale di Fisica Nucleare-LNS Catania, 95125 Catania, Italy; [email protected] (G.A.P.C.); [email protected] (G.C.) 4 Dipartimento di Chirurgia Generale e Specialità Medico-Chirurgiche, Università di Catania, 95125 Catania, Italy; [email protected] (A.R.); [email protected] (T.A.); [email protected] (A.L.) 5 Dipartimento di Oftalmologia, Università di Torino, 10124 Torino, Italy; [email protected] 6 Dipartimento