(Lamiaceae) from Macedonia with HPLC-UV DAD
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity
applied sciences Article The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity Małgorzata Ziarno 1,* , Mariola Kozłowska 2 , Iwona Scibisz´ 3 , Mariusz Kowalczyk 4 , Sylwia Pawelec 4 , Anna Stochmal 4 and Bartłomiej Szleszy ´nski 5 1 Division of Milk Technology, Department of Food Technology and Assessment, Institute of Food Science, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland 2 Department of Chemistry, Institute of Food Science, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland; [email protected] 3 Division of Fruit, Vegetable and Cereal Technology, Department of Food Technology and Assessment, Institute of Food Science, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland; [email protected] 4 Department of Biochemistry and Crop Quality, Institute of Soil Science and Plant Cultivation, State Research Institute, 24-100 Puławy, Poland; [email protected] (M.K.); [email protected] (S.P.); [email protected] (A.S.) 5 Institute of Horticultural Sciences, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-225-937-666 Abstract: This study aimed to investigate the effect of plant extracts (valerian Valeriana officinalis L., sage Salvia officinalis L., chamomile Matricaria chamomilla L., cistus Cistus L., linden blossom Tilia L., ribwort plantain Plantago lanceolata L., marshmallow Althaea L.) on the activity and growth of lactic acid bacteria (LAB) during the fermentation and passage of milk through a digestive system model. Citation: Ziarno, M.; Kozłowska, M.; The tested extracts were also characterized in terms of their content of polyphenolic compounds and Scibisz,´ I.; Kowalczyk, M.; Pawelec, S.; antioxidant activity. -

Sideritis Clandestina (Bory & Chaub.) Hayek; Sideritis Raeseri Boiss
2 February 2016 EMA/HMPC/39455/2015 Committee on Herbal Medicinal Products (HMPC) Assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba Final Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use) Herbal substances (binomial scientific name of Sideritis scardica Griseb.; Sideritis clandestina the plant, including plant part) (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba Herbal preparation Comminuted herbal substance Pharmaceutical form Comminuted herbal substance as herbal tea for oral use Rapporteur I. Chinou Peer-reviewer B. Kroes 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ................................................................................................................... 2 1. Introduction ....................................................................................................................... 4 1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof .. 4 1.2. Search and assessment methodology ..................................................................... 8 2. Data on -

Shilin Yang Doctor of Philosophy
PHYTOCHEMICAL STUDIES OF ARTEMISIA ANNUA L. THESIS Presented by SHILIN YANG For the Degree of DOCTOR OF PHILOSOPHY of the UNIVERSITY OF LONDON DEPARTMENT OF PHARMACOGNOSY THE SCHOOL OF PHARMACY THE UNIVERSITY OF LONDON BRUNSWICK SQUARE, LONDON WC1N 1AX ProQuest Number: U063742 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U063742 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKNOWLEDGEMENT I wish to express my sincere gratitude to Professor J.D. Phillipson and Dr. M.J.O’Neill for their supervision throughout the course of studies. I would especially like to thank Dr. M.F.Roberts for her great help. I like to thank Dr. K.C.S.C.Liu and B.C.Homeyer for their great help. My sincere thanks to Mrs.J.B.Hallsworth for her help. I am very grateful to the staff of the MS Spectroscopy Unit and NMR Unit of the School of Pharmacy, and the staff of the NMR Unit, King’s College, University of London, for running the MS and NMR spectra. -

Phenolic Profiling of Veronica Spp. Grown in Mountain, Urban and Sand Soil Environments
CORE Metadata, citation and similar papers at core.ac.uk Provided by Biblioteca Digital do IPB Phenolic profiling of Veronica spp. grown in mountain, urban and sand soil environments João C.M. Barreiraa,b,c, Maria Inês Diasa,c, Jelena Živkovićd, Dejan Stojkoviće, Marina Sokoviće, Celestino Santos-Buelgab,*, Isabel C.F.R. Ferreiraa,* aCIMO/Escola Superior Agrária, Instituto Politécnico de Bragança, Apartado 1172, 5301-855 Bragança, Portugal. bGIP-USAL, Facultad de Farmacia, Universidad de Salamanca, Campus Miguel de Unamuno, 37007 Salamanca, Spain. cREQUIMTE/Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, Rua Jorge Viterbo Ferreira, nº 228, 4050-313 Porto, Portugal. dInstitute for Medicinal Plant Research “Dr. Josif Pančić”, Tadeuša Košćuška 1, 11000 Belgrade, Serbia. eDepartment of Plant Physiology, Institute for Biological Research “Siniša Stanković”, University of Belgrade, Bulevar Despota Stefana 142, 11000 Belgrade, Serbia. * Authors to whom correspondence should be addressed (Isabel C.F.R. Ferreira; e-mail: [email protected], telephone +351273303219, fax +351273325405; e-mail: Celestino Santos- Buelga: [email protected]; telephone +34923294537; fax +34923294515). 1 Abstract Veronica (Plantaginaceae) genus is widely distributed in different habitats. Phytochemistry studies are increasing because most metabolites with pharmacological interest are obtained from plants. The phenolic compounds of V. montana, V. polita and V. spuria were tentatively identified by HPLC-DAD-ESI/MS. The phenolic profiles showed that flavones were the major compounds (V. montana: 7 phenolic acids, 5 flavones, 4 phenylethanoids and 1 isoflavone; V. polita: 10 flavones, 5 phenolic acids, 2 phenylethanoids, 1 flavonol and 1 isoflavone; V. spuria: 10 phenolic acids, 5 flavones, 2 flavonols, 2 phenylethanoids and 1 isoflavone), despite the overall predominance of flavones. -

Herbal Principles in Cosmetics Properties and Mechanisms of Action Traditional Herbal Medicines for Modern Times
Traditional Herbal Medicines for Modern Times Herbal Principles in Cosmetics Properties and Mechanisms of Action Traditional Herbal Medicines for Modern Times Each volume in this series provides academia, health sciences, and the herbal medicines industry with in-depth coverage of the herbal remedies for infectious diseases, certain medical conditions, or the plant medicines of a particular country. Series Editor: Dr. Roland Hardman Volume 1 Shengmai San, edited by Kam-Ming Ko Volume 2 Rasayana: Ayurvedic Herbs for Rejuvenation and Longevity, by H.S. Puri Volume 3 Sho-Saiko-To: (Xiao-Chai-Hu-Tang) Scientific Evaluation and Clinical Applications, by Yukio Ogihara and Masaki Aburada Volume 4 Traditional Medicinal Plants and Malaria, edited by Merlin Willcox, Gerard Bodeker, and Philippe Rasoanaivo Volume 5 Juzen-taiho-to (Shi-Quan-Da-Bu-Tang): Scientific Evaluation and Clinical Applications, edited by Haruki Yamada and Ikuo Saiki Volume 6 Traditional Medicines for Modern Times: Antidiabetic Plants, edited by Amala Soumyanath Volume 7 Bupleurum Species: Scientific Evaluation and Clinical Applications, edited by Sheng-Li Pan Traditional Herbal Medicines for Modern Times Herbal Principles in Cosmetics Properties and Mechanisms of Action Bruno Burlando, Luisella Verotta, Laura Cornara, and Elisa Bottini-Massa Cover art design by Carlo Del Vecchio. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2010 by Taylor and Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-13: 978-1-4398-1214-3 (Ebook-PDF) This book contains information obtained from authentic and highly regarded sources. -

Cushnie TPT, Lamb AJ. Antimicrobial Activity of Flavonoids. International Journal of Antimicrobial Agents, 2005. 26(5):343-356
Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 2005. 26(5):343-356. PMID: 16323269 DOI: 10.1016/j.ijantimicag.2005.09.002 The journal article above is freely available from the publishers at: http://www.idpublications.com/journals/PDFs/IJAA/ANTAGE_MostCited_1.pdf and also... http://www.ijaaonline.com/article/S0924-8579(05)00255-4/fulltext Errata for the article (typesetting errors by Elsevier Ireland) are freely available from the publishers at: http://www.ijaaonline.com/article/S0924-8579(05)00352-3/fulltext and also... http://www.sciencedirect.com/science/article/pii/S0924857905003523 International Journal of Antimicrobial Agents 26 (2005) 343–356 Review Antimicrobial activity of flavonoids T.P. Tim Cushnie, Andrew J. Lamb ∗ School of Pharmacy, The Robert Gordon University, Schoolhill, Aberdeen AB10 1FR, UK Abstract Flavonoids are ubiquitous in photosynthesising cells and are commonly found in fruit, vegetables, nuts, seeds, stems, flowers, tea, wine, propolis and honey. For centuries, preparations containing these compounds as the principal physiologically active constituents have been used to treat human diseases. Increasingly, this class of natural products is becoming the subject of anti-infective research, and many groups have isolated and identified the structures of flavonoids possessing antifungal, antiviral and antibacterial activity. Moreover, several groups have demonstrated synergy between active flavonoids as well as between flavonoids and existing chemotherapeutics. Reports of activity in the field of antibacterial flavonoid research are widely conflicting, probably owing to inter- and intra-assay variation in susceptibility testing. However, several high-quality investigations have examined the relationship between flavonoid structure and antibacterial activity and these are in close agreement. -

Distribution of Flavonoids Among Malvaceae Family Members – a Review
Distribution of flavonoids among Malvaceae family members – A review Vellingiri Vadivel, Sridharan Sriram, Pemaiah Brindha Centre for Advanced Research in Indian System of Medicine (CARISM), SASTRA University, Thanjavur, Tamil Nadu, India Abstract Since ancient times, Malvaceae family plant members are distributed worldwide and have been used as a folk remedy for the treatment of skin diseases, as an antifertility agent, antiseptic, and carminative. Some compounds isolated from Malvaceae members such as flavonoids, phenolic acids, and polysaccharides are considered responsible for these activities. Although the flavonoid profiles of several Malvaceae family members are REVIEW REVIEW ARTICLE investigated, the information is scattered. To understand the chemical variability and chemotaxonomic relationship among Malvaceae family members summation of their phytochemical nature is essential. Hence, this review aims to summarize the distribution of flavonoids in species of genera namely Abelmoschus, Abroma, Abutilon, Bombax, Duboscia, Gossypium, Hibiscus, Helicteres, Herissantia, Kitaibelia, Lavatera, Malva, Pavonia, Sida, Theobroma, and Thespesia, Urena, In general, flavonols are represented by glycosides of quercetin, kaempferol, myricetin, herbacetin, gossypetin, and hibiscetin. However, flavonols and flavones with additional OH groups at the C-8 A ring and/or the C-5′ B ring positions are characteristic of this family, demonstrating chemotaxonomic significance. Key words: Flavones, flavonoids, flavonols, glycosides, Malvaceae, phytochemicals INTRODUCTION connate at least at their bases, but often forming a tube around the pistils. The pistils are composed of two to many connate he Malvaceae is a family of flowering carpels. The ovary is superior, with axial placentation, with plants estimated to contain 243 genera capitate or lobed stigma. The flowers have nectaries made with more than 4225 species. -

Serbian Journal of Experimental and Clinical Research Vol12
Editor-in-Chief Slobodan Janković Co-Editors Nebojša Arsenijević, Miodrag Lukić, Miodrag Stojković, Milovan Matović, Slobodan Arsenijević, Nedeljko Manojlović, Vladimir Jakovljević, Mirjana Vukićević Board of Editors Ljiljana Vučković-Dekić, Institute for Oncology and Radiology of Serbia, Belgrade, Serbia Dragić Banković, Faculty for Natural Sciences and Mathematics, University of Kragujevac, Kragujevac, Serbia Zoran Stošić, Medical Faculty, University of Novi Sad, Novi Sad, Serbia Petar Vuleković, Medical Faculty, University of Novi Sad, Novi Sad, Serbia Philip Grammaticos, Professor Emeritus of Nuclear Medicine, Ermou 51, 546 23, Th essaloniki, Macedonia, Greece Stanislav Dubnička, Inst. of Physics Slovak Acad. Of Sci., Dubravska cesta 9, SK-84511 Bratislava, Slovak Republic Luca Rosi, SAC Istituto Superiore di Sanita, Vaile Regina Elena 299-00161 Roma, Italy Richard Gryglewski, Jagiellonian University, Department of Pharmacology, Krakow, Poland Lawrence Tierney, Jr, MD, VA Medical Center San Francisco, CA, USA Pravin J. Gupta, MD, D/9, Laxminagar, Nagpur – 440022 India Winfried Neuhuber, Medical Faculty, University of Erlangen, Nuremberg, Germany Editorial Staff Ivan Jovanović, Gordana Radosavljević, Nemanja Zdravković Vladislav Volarević Management Team Snezana Ivezic, Milan Milojevic, Bojana Radojevic, Ana Miloradovic Corrected by Scientifi c Editing Service “American Journal Experts” Design PrstJezikIostaliPsi - Miljan Nedeljković Print Medical Faculty, Kragujevac Indexed in EMBASE/Excerpta Medica, Index Copernicus, BioMedWorld, KoBSON, -
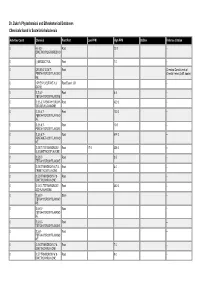
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals Found in Scutellaria Baicalensis
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals found in Scutellaria baicalensis Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 (+)-5,5'- Root 22.0 -- DIMETHOXYLARICIRESINO L 0 (-)-ERODICTYOL Root 7.0 -- 0 (2R,3R)-2',3,5,6',7- Root Chemical Constituents of PENTAHYDROXYFLAVONO Oriental Herbs (3 diff. books) NE 0 1-PHENYL-BUTANE-1,3- Root Essent. Oil -- DIONE 0 2',3',5,7- Root 6.4 -- TETRAHYDROXYFLAVONE 0 2',3,5,6',7-PENTAHYDROXY- Root 420.0 -- 2(R),3(R)-FLAVANONE 0 2',3,5,6',7- Root 120.0 -- PENTAHYDROXYFLAVANO NE 0 2',3,5,6',7- Root 10.0 -- PENTAHYDROXYFLAVONE 0 2',3,5,6',7- Root 674.0 -- PENTAMETHOXYFLAVANO NE 0 2',5,5',7-TETRAHYDROXY- Root 17.0 356.0 -- 6',8-DIMETHOXYFLAVONE 0 2',5,5',7- Root 3.5 -- TETRAHYDROXYFLAVONE 0 2',5,5'-TRIHYDROXY-6,7,8- Root 4.0 -- TRIMETHOXYFLAVONE 0 2',5,5'-TRIHYDROXY-7,8- Root -- DIMETHOXYFLAVONE 0 2',5,6',7-TETRAHYDROXY- Root 380.0 -- 2(S)-FLAVANONE 0 2',5,6',7- Stem -- TETRAHYDROXYFLAVANO NE 0 2',5,6',7- Root -- TETRAHYDROXYFLAVANO NE 0 2',5,6',7- Root -- TETRAHYDROXYFLAVONE 0 2',5,6'- Root -- TETRAHYDROXYFLAVANO NE 0 2',5,6'-TRIHYDROXY-7,8- Root 7.0 -- DIMETHOXYFLAVONE 0 2',5,7-TRIHYDROXY-6',8- Root 9.0 -- DIMETHOXYFLAVONE Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 2',5,7-TRIHYDROXY-6',8- Root 7.0 -- TRIMETHOXYFLAVONE 0 2',5,7-TRIHYDROXY-6'- Root 4.0 -- METHOXYFLAVONE 0 2',5,7-TRIHYDROXY-8- Root 6.0 -- METHOXYFLAVONE 0 2',5,7- Root 6.0 -- TRIHYDROXYFLAVONE 0 2',5,8-TRIHYDROXY-6,7- Root 200.0 -- DIMETHOXYFLAVONE 0 2'- Root -- METHYLSKULLCAPFLAVO NE 0 2(S),2',5,6',7- Root 230.0 -- TETRAHYDROXYFLAVANO NE 0 2,2',4',6-TETRAHYDROXY- Root 5.0 -- 6'-METHOXYCHALCONE 1 2,3-DIHYDROBENZOFURAN Root Essent. -

Exudate Flavonoids in Some Gnaphalieae and Inuleae (Asteraceae) Eckhard Wollenwebera,*, Matthias Christa, R
Exudate Flavonoids in Some Gnaphalieae and Inuleae (Asteraceae) Eckhard Wollenwebera,*, Matthias Christa, R. Hugh Dunstanb, James N. Roitmanc, and Jan F. Stevensd a Institut für Botanik der TU Darmstadt, Schnittspahnstrasse 4, D-64287 Darmstadt, Germany. Fax: 0049-6151/164630. E-mail: [email protected] b School of Environmental and Life Sciences, The University of Newcastle, Callaghan, NSW 2308, Australia c Western Regional Research Center, USDA-ARS, 800 Buchanan Street, Albany, CA 94710, U.S.A. d Department of Pharmaceutical Sciences, 203 Pharmacy Building, Oregon State University, Corvallis, OR 97331, U.S.A. * Author for correspondance and reprint requests Z. Naturforsch. 60c, 671Ð678 (2005); received May 19, 2005 Three members of the tribe Gnaphalieae and six members of the tribe Inuleae (Astera- ceae) were analyzed for their exudate flavonoids. Whereas some species exhibit rather trivial flavonoids, others produce rare compounds. Spectral data of rare flavonoids are reported and their structural identification is discussed. 6-Oxygenation of flavonols is a common feature of two Inula species and Pulicaria sicula. By contrast, flavonoids with 8-oxygenation, but lacking 6-oxygenation, are common in two out of three Gnaphalieae species examined. In addition, B-ring deoxyflavonoids are abundantly present in the leaf exudates of Helichrysum italicum (Gnaphalieae). These distinctive features of the two Asteraceae tribes are in agreement with previous flavonoid surveys of these and related taxa. Key words: Gnaphalieae, Inuleae, Flavonoids Introduction in the flowering stage between October 1997 and Plants belonging to the sunflower family are August 2004. Inula britannica L. was collected in well-known to produce a wealth of flavonoid agly- August 2000 on the bank of the river Elbe near cones (Bohm and Stuessy, 2001). -

Organ-Specific Metabolic Shifts of Flavonoids in Scutellaria
molecules Article Organ-Specific Metabolic Shifts of Flavonoids in Scutellaria baicalensis at Different Growth and Development Stages Jingyuan Xu ID , Yilan Yu, Ruoyun Shi, Guoyong Xie, Yan Zhu, Gang Wu and Minjian Qin * ID Department of Resources Science of Traditional Chinese Medicines, School of Traditional Chinese Pharmacy and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing 210009, China; [email protected] (J.X.); [email protected] (Y.Y.); [email protected] (R.S.); [email protected] (G.X.); [email protected] (Y.Z.); [email protected] (G.W.) * Correspondence: [email protected]; Tel.: +86-025-8618-5130 Received: 24 January 2018; Accepted: 14 February 2018; Published: 15 February 2018 Abstract: Scutellaria baicalensis Georgi is a traditional Chinese herbal medicine mainly containing flavonoids that contribute to its bioactivities. In this study, the distributions and dynamic changes of flavonoid levels in various organs of S. baicalensis at different development stages were investigated by UHPLC-QTOF-MS/MS and HPLC-DAD methods. The results indicated that the metabolic profiles of S. baicalensis changed with growth and development. During the initial germination stage, the seeds mainly contained flavonols. With growth, the main kinds of flavonoids in S. baicalensis changed from flavonols to flavanones and flavones. The results also revealed that the accumulation of flavonoids in S. baicalensis is organ-specific. The flavones without 40-OH groups mainly accumulate in the root and the flavanones mainly accumulate in aerial organs. Dynamic accumulation analysis showed that the main flavonoids in the root of S. baicalensis accumulated rapidly before the full-bloom stage, then changed to a small extent. -

Print This Article
International Journal of Phytomedicine 8 (2016) 139-175 http://www.arjournals.org/index.php/ijpm/index Review Article ISSN: 0975-0185 A Detailed Overview of Medicinal Plants Having Hypoglycemic Activity Sandra Celine1, Shawn Tomy1, Ujwala TK1, Sam Johnson1, Udaya Chander J1* *Corresponding author: A b s t r a c t Diabetes mellitus represents a spectrum of metabolic disorder, which has become one of the Udaya Chander major public health concerns worldwide. Diabetes mellitus has emerged as a third leading killer after cancer and cardiovascular/cerebrovascular diseases and India has a distinction of having 1Pharm D Intern, RVS College of largest number of diabetics in world second to China. Herbal medicine for treating chronic Pharmaceutical Sciences, Coimbatore, Tamil diseases, especially diabetes has gained an exponential growth in the last few years and both Nadu, India developing and developed countries are adopting herbal drugs for treatment of diabetes mellitus. The World Health Organization (WHO) has listed 21,000 plants, which are used for medicinal purposes around the world. The WHO has defined herbal medicines as finished labelled medicinal products that contain aerial or underground parts of the plants or other plant material or combination thereof as active ingredients, whether in crude state or as plant preparations. This review attempts to present the profiles of plants with hypoglycemic properties, reported in the literature with proper categorization according to the botanical name, family, parts used, chemical constituents, and its other uses. Relevant medical databases and websites were searched. To qualify for inclusion, the herbs should have confirmed hypoglycemic potential. Other criteria for inclusion are: published in English and peer-reviewed journals.