Transthoracic Echocardiography in Children – (0523)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Disease in Children.Pdf
Heart Disease in Children Richard U. Garcia, MD*, Stacie B. Peddy, MD KEYWORDS Congenital heart disease Children Primary care Cyanosis Chest pain Heart murmur Infective endocarditis KEY POINTS Fetal and neonatal diagnosis of congenital heart disease (CHD) has improved the out- comes for children born with critical CHD. Treatment and management of CHD has improved significantly over the past 2 decades, allowing more children with CHD to grow into adulthood. Appropriate diagnosis and treatment of group A pharyngitis and Kawasaki disease in pe- diatric patients mitigate late complications. Chest pain, syncope, and irregular heart rhythm are common presentations in primary care. Although typically benign, red flag symptoms/signs should prompt a referral to car- diology for further evaluation. INTRODUCTION The modern incidence of congenital heart disease (CHD) has been reported at 6 to 11.1 per 1000 live births.1,2 The true incidence is likely higher because many miscar- riages are due to heart conditions incompatible with life. The unique physiology of CHD, the constantly developing nature of children, the differing presenting signs and symptoms, the multiple palliative or corrective surgeries, and the constant devel- opment of new strategies directed toward improving care in this population make pe- diatric cardiology an exciting field in modern medicine. THE FETAL CIRCULATION AND TRANSITION TO NEONATAL LIFE Cardiovascular morphogenesis is a complex process that transforms an initial single- tube heart to a 4-chamber heart with 2 separate outflow tracts. Multiple and Disclosure Statement: All Authors take responsibility for all aspects of the reliability and freedom from bias of the information presented and their discussed interpretation. -

Chest Pain in Children and Adolescents Surendranath R
Article cardiology Chest Pain in Children and Adolescents Surendranath R. Veeram Objectives After completing this article, readers should be able to: Reddy, MD,* Harinder R. Singh, MD* 1. Enumerate the most common causes of chest pain in pediatric patients. 2. Differentiate cardiac chest pain from that of noncardiac cause. 3. Describe the detailed evaluation of a pediatric patient who has chest pain. Author Disclosure 4. Screen and identify patients who require a referral to a pediatric cardiologist or other Drs Veeram Reddy specialist. and Singh have 5. Explain the management of the common causes of pediatric chest pain. disclosed no financial relationships relevant Case Studies to this article. This Case 1 commentary does not During an annual physical examination, a 12-year-old girl complains of intermittent chest contain a discussion pain for the past 5 days that localizes to the left upper sternal border. The pain is sharp and of an unapproved/ stabbing, is 5/10 in intensity, increases with deep breathing, and lasts for less than 1 minute. investigative use of a The patient has no history of fever, cough, exercise intolerance, palpitations, dizziness, or commercial product/ syncope. On physical examination, the young girl is in no pain or distress and has normal vital signs for her age. Examination of her chest reveals no signs of inflammation over the sternum device. or rib cage. Palpation elicits mild-to-moderate tenderness over the left second and third costochondral junctions. The patient reports that the pain during the physical examination is similar to the chest pain she has experienced for the past 5 days. -

Chest Pain in Pediatrics
PEDIATRIC CARDIOLOGY 0031-3955/99 $8.00 + .OO CHEST PAIN IN PEDIATRICS Keith C. Kocis, MD, MS Chest pain is an alarming complaint in children, leading an often frightened and concerned family to a pediatrician or emergency room and commonly to a subsequent referral to a pediatric cardiologist. Because of the well-known associ- ation of chest pain with significant cardiovascular disease and sudden death in adult patients, medical personnel commonly share heightened concerns over pediatric patients presenting with chest pain. Although the differential diagnosis of chest pain is exhaustive, chest pain in children is least likely to be cardiac in origin. Organ systems responsible for causing chest pain in children include*: Idiopathic (12%-85%) Musculoskeletal (15%-31%) Pulmonary (12%-21%) Other (4%-21%) Psychiatric (5%-17%) Gastrointestinal (4'/0-7%) Cardiac (4%4%) Furthermore, chest pain in the pediatric population is rareZy associated with life-threatening disease; however, when present, prompt recognition, diagnostic evaluation, and intervention are necessary to prevent an adverse outcome. This article presents a comprehensive list of differential diagnostic possibilities of chest pain in pediatric patients, discusses the common causes in further detail, and outlines a rational diagnostic evaluation and treatment plan. Chest pain, a common complaint of pediatric patients, is often idiopathic in etiology and commonly chronic in nature. In one study,67 chest pain accounted for 6 in 1000 visits to an urban pediatric emergency room. In addition, chest pain is the second most common reason for referral to pediatric cardiologist^.^, 23, 78 Chest pain is found equally in male and female patients, with an average *References 13, 17, 23, 27, 32, 35, 44, 48, 49, 63-67, 74, and 78. -
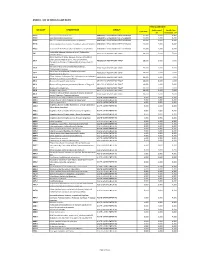
Annex 1: List of Medical Case Rates
ANNEX 1. LIST OF MEDICAL CASE RATES FIRST CASE RATE ICD CODE DESCRIPTION GROUP Professional Health Care Case Rate Fee Institution Fee P91.3 Neonatal cerebral irritability ABNORMAL SENSORIUM IN THE NEWBORN 12,000 3,600 8,400 P91.4 Neonatal cerebral depression ABNORMAL SENSORIUM IN THE NEWBORN 12,000 3,600 8,400 P91.6 Hypoxic ischemic encephalopathy of newborn ABNORMAL SENSORIUM IN THE NEWBORN 12,000 3,600 8,400 P91.8 Other specified disturbances of cerebral status of newborn ABNORMAL SENSORIUM IN THE NEWBORN 12,000 3,600 8,400 P91.9 Disturbance of cerebral status of newborn, unspecified ABNORMAL SENSORIUM IN THE NEWBORN 12,000 3,600 8,400 Peritonsillar abscess; Abscess of tonsil; Peritonsillar J36 ABSCESS OF RESPIRATORY TRACT 10,000 3,000 7,000 cellulitis; Quinsy Other diseases of larynx; Abscess of larynx; Cellulitis of larynx; Disease NOS of larynx; Necrosis of larynx; J38.7 ABSCESS OF RESPIRATORY TRACT 10,000 3,000 7,000 Pachyderma of larynx; Perichondritis of larynx; Ulcer of larynx Retropharyngeal and parapharyngeal abscess; J39.0 ABSCESS OF RESPIRATORY TRACT 10,000 3,000 7,000 Peripharyngeal abscess Other abscess of pharynx; Cellulitis of pharynx; J39.1 ABSCESS OF RESPIRATORY TRACT 10,000 3,000 7,000 Nasopharyngeal abscess Other diseases of pharynx; Cyst of pharynx or nasopharynx; J39.2 ABSCESS OF RESPIRATORY TRACT 10,000 3,000 7,000 Oedema of pharynx or nasopharynx J85.1 Abscess of lung with pneumonia ABSCESS OF RESPIRATORY TRACT 10,000 3,000 7,000 J85.2 Abscess of lung without pneumonia; Abscess of lung NOS ABSCESS OF RESPIRATORY -

Identifying and Treating Chest Pain
Identifying and Treating Chest Pain The Congenital Heart Collaborative Cardiac Chest Pain University Hospitals Rainbow Babies & Children’s Hospital Chest pain due to a cardiac condition is rare in children and and Nationwide Children’s Hospital have formed an innovative adolescents, with a prevalence of less than 5 percent. The affiliation for the care of patients with congenital heart disease cardiac causes of chest pain include inflammation, coronary from fetal life to adulthood. The Congenital Heart Collaborative insufficiency, tachyarrhythmias, left ventricular outflow tract provides families with access to one of the most extensive and obstruction and connective tissue abnormalities. experienced heart teams – highly skilled in the delivery of quality clinical services, novel therapies and a seamless continuum of care. Noncardiac Chest Pain Noncardiac chest pain is, by far, the most common cause of chest pain in children and adolescents, accounting for 95 percent of Pediatric Chest Pain concerns. Patients are often unnecessarily referred to a pediatric In pediatrics, chest pain has a variety of symptomatic levels and cardiologist for symptoms. This causes increased anxiety and causes. It can range from a sharp stab to a dull ache; a crushing distress within the family. Noncardiac causes of chest pain are or burning sensation; or even pain that travels up to the neck, musculoskeletal, pulmonary, gastrointestinal and miscellaneous. jaw and back. Chest pain can be cause for alarm in both patients The most common cause of chest pain in children and and parents, and it warrants careful examination and treatment. adolescents is musculoskeletal or chest-wall pain. Pediatric chest pain can be broadly classified as cardiac chest pain Reassurance, rest and analgesia are the primary treatments or noncardiac chest pain. -

Kounis Syndrome: a Forgotten Cause of References Chest Pain/ Cardiac Chest Pain in Children 1
382 Editöre Mektuplar Letters to the Editor Kounis syndrome: A forgotten cause of References chest pain/ Cardiac chest pain in children 1. Çağdaş DN, Paç FA. Cardiac chest pain in children. Anadolu Kardiyol Derg 2009;9:401-6. Kounis sendromu: Göğüs ağrısının unutulan bir sebebi/ 2. Coleman WL. Recurrent chest pain in children. Pediatr Clin North Am Çocuklarda kardiyak göğüs ağrısı 1984;31:1007-26. 3. Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol 2006; 7:7-14. Dear Editor, 4. Biteker M, Duran NE, Biteker F, Civan HA, Gündüz S, Gökdeniz T, et al. Kounis syndrome: first series in Turkish patients. Anadolu Kardiyol Derg I read with interest the article “Cardiac chest pain in children” by 2009;9:59-60. Çağdaş et al. (1) which has retrospectively evaluated 120 children 5. Biteker M. A new classification of Kounis syndrome. Int J Cardiol 2010 Jun admitted to a pediatric cardiology clinic with chest pain. Although chest 7. [Epub ahead of print]. pain in children is rarely reported to be associated with cardiac 6. Biteker M, Duran NE, Ertürk E, Aykan AC, Civan HA, et al. Kounis Syndrome diseases in the literature (2) authors have found that 52 of the patients secondary to amoxicillin/clavulanic acid use in a child. Int J Cardiol 2009;136:e3-5. (42.5%) had cardiac diseases and 28 (23.3%) of these patients’ cardiac 7. Biteker M, Ekşi Duran N, Sungur Biteker F, Ayyıldız Civan H, Kaya H, diseases were thought to directly cause their chest pain. -

Keepthebeatconference 2014
Texas Children’s HOSPITAL keepthebeatconference 2014 Thursday, May 1 – Saturday, May 3, 2014 Presented by Texas Children’s Hospital and Baylor College of Medicine Texas Children’s Pavilion for Women 4th Floor Conference Center n 6621 Fannin Street n Houston, TX 77030 THIS year’S PROGRAM INCLUDES TWO EXCITING TRACKS! INPATIENT CARDIOLOGY OUTPATIENT CARDIOLOGY TRACK TRACK View program and register online at View program and register online at BaylorCME.org/CME/1489-MI BaylorCME.org/CME/1489-MO HEART MURMUR WORKSHOP MAY 3 View page 8 for more information. Co-sponsored by Texas Children’s Hospital and Baylor College of Medicine PLANNING COMMITTEE Silvana M. Lawrence, MD, PhD Director, Community and Program Development, Texas Children’s Hospital Associate Professor of Pediatrics, Baylor Collge of Medicine William B. Kyle, MD Pediatric Cardiologist, Texas Children’s Hospital Assistant Professor of Pediatrics, Baylor Collge of Medicine Priscila P. Reid, RN, FNP-C, PNP-AC Nurse Practitioner, Texas Children’s Hospital Instructor of Pediatrics, Baylor Collge of Medicine GUEST FACULTY Jane Burns, MD Chitra Ravishankar, MD Professor of Pediatrics Assistant Professor of Pediatrics University of California - San Diego University of Pennsylvania Ganga Krishnamurthy, MD Marah N. Short Assistant Professor of Pediatrics Senior Staff Researcher Columbia University James A. Baker, III Institute for Public Policy Rice University BAYLOR COLLEGE OF MEDICINE FACULTY Steven A. Abrams, MD Jeffrey S. Heinle, MD Christina Y. Miyake, MD Professor of Pediatrics Associate Professor of Surgery Assistant Professor of Pediatrics Hugh D. Allen, MD Aamir Jeewa, MD Antonio R. Mott, MD Professor of Pediatrics Assistant Professor of Pediatrics Associate Professor of Pediatrics Carolyn A. -

Ten Leading Causes of Death by Age Group,Race/Ethnicity, and Gender
Ten Leading Causes of Death by Age Group, Race/Ethnicity, and Gender Suburban Cook County 2006-2008 Suggested Citation: Cook County Department of Public Health. (2013). Ten Leading Causes of Death Tables 2008. Oak Forest, IL: Community Epidemiology and Health Planning Unit Table of Contents All Suburban Cook County: All Deaths By Gender: Females Males By Race/Ethnicity and Gender: Hispanic Hispanic Females Hispanic Males Non Hispanic Asian/Pacific Islander Non Hispanic Asian/Pacific Islander Females Non Hispanic Asian/Pacific Islander Males Non Hispanic Black Non Hispanic Black Females Non Hispanic Black Males Non Hispanic White Non Hispanic White Females Non Hispanic White Males By District: Cook County Department of Public Health Jurisdiction North District West District Southwest District South District Resources: District Map Rankable Causes of Death Rankable Causes of Infant Death 10 Leading Causes of Death by Age Group, Suburban Cook County, 2006-2008 (Counts/Percent of Total Deaths) All Deaths Rank <1 1-4 5-17 18-24 25-34 35-44 45-54 55-64 65+ Total Unintentional Unintentional Unintentional Unintentional Malignant Malignant Malignant Short Gestation Heart Disease Heart Disease 1 Injury Injury Injury Injury Neoplasms Neoplasms Neoplasms 170 24.9% 24 23.8% 57 24.6% 196 37.0% 281 34.4% 300 19.2% 1,251 31.7% 2,489 38.9% 13,422 28.2% 16,345 26.4% Congenital Congenital Unintentional Malignant Malignant Homicide Homicide Homicide Heart Disease Heart Disease 2 Anomalies Anomalies Injury Neoplasms Neoplasms 127 18.6% 9 8.9% 44 19.0% 135 -

Effectiveness of Screening for Life-Threatening Chest Pain in Children
Effectiveness of Screening for Life-Threatening Chest Pain in Children WHAT’S KNOWN ON THIS SUBJECT: Chest pain in children is an AUTHORS: Susan F. Saleeb, MD, Wing Yi V. Li, BA, Shira Z. extremely frequent complaint, with generally benign causes. Warren, BA, and James E. Lock, MD Referrals to cardiologists are increasing in volume, although the Department of Cardiology, Children’s Hospital Boston and frequency of cardiac causes is exceedingly low. Harvard Medical School, Harvard University, Boston, Massachusetts WHAT THIS STUDY ADDS: This study demonstrates that thorough KEY WORDS history assessments, physical examinations, and chest pain, standardized clinical assessment and management electrocardiograms can be used effectively in initial screening to plan, congenital heart disease determine when higher-level care and testing are needed. This ABBREVIATIONS technique allowed for no cardiac deaths over a 10-year period. CP—chest pain SCAMP—standardized clinical assessment and management plan ECG—electrocardiogram EST—exercise stress test SVT—supraventricular tachycardia abstract ICD-9—International Classification of Diseases, Ninth Revision www.pediatrics.org/cgi/doi/10.1542/peds.2011-0408 OBJECTIVE: We sought to determine the incidence of sudden cardiac doi:10.1542/peds.2011-0408 death among patients discharged from the cardiology clinic with pre- Accepted for publication Jul 29, 2011 sumed noncardiac chest pain (CP). Address correspondence to Susan F. Saleeb, MD, Children’s METHODS: The records of children Ͼ6 years of age who presented to Hospital Boston, Department of Cardiology, 300 Longwood Ave, Children’s Hospital Boston between January 1, 2000, and December 31, Boston, MA 02115. E-mail: [email protected] 2009, with a complaint of CP were reviewed for demographic features, PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275). -

Chest Pain in Children: It's Not All Heart • None
6/18/2019 Disclosures Chest Pain in Children: It's Not All Heart • none • Cathy S. Woodward, DNP, RN, PNP-AC • Professor of Pediatrics • UT Health -San Antonio Objectives Chest Pain •List the most common causes of benign chest pain in children. •Discuss the differential for children presenting with acute chest pain. •Describe the must-not-miss assessments in children with serious chest pain. Chest Pain Incidence of CP complaints •3700 kids evaluated for CP only 1% related to cardiac cause. •Musculoskeletal •Pulmonary •Gastrointestinal •Anxiety •Unknown cause 1 6/18/2019 Musculoskeletal CP •Brief sharp chest pain •Worse with deep breathing and movement •History of trauma, fall, new exercise, cough Musculoskeletal Causes of CP Slipping Rib Syndrome •31% of children who see cardiologists •Described in 1919 •Connective, bony and muscular tissue •Hypermobility of ant ends of ribs 8-10 •Precordial catch – short duration, unclear •Precipitating cause – cough, exercise, etiology trauma •Costochondritis •Pain acute and is reproducible •Trauma •Hooking maneuver • Slipping rib syndrome Pulmonary Causes of CP Pneumothorax •Chest Pain with •Asthma SOB •Pneumonia/Pleuritis •Trauma or •Chronic cough spontaneous •Pneumothorax •No breath •Acute Chest Syndrome sounds on affected side 2 6/18/2019 Acute Chest Syndrome Gastrointestinal Causes of CP •Major cause of morbidity and mortality for •Esophageal reflux – burning pain, center of children with Sickle Cell Disease – vaso chest, pain increased or decreased by certain occlusive crisis foods and body position -

Helping Mothers Defend Their Decision to Breastfeed
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2015 Helping Mothers Defend their Decision to Breastfeed Kandis Natoli University of Central Florida Part of the Nursing Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Natoli, Kandis, "Helping Mothers Defend their Decision to Breastfeed" (2015). Electronic Theses and Dissertations, 2004-2019. 1392. https://stars.library.ucf.edu/etd/1392 HELPING MOTHERS DEFEND THEIR DECISION TO BREASTFEED by KANDIS M. NATOLI M.S.N. University of Central Florida, 2006 B.S.N. University of Central Florida, 2005 A dissertation submitted in partial fulfillment of the requirements for the Degree of Doctor of Philosophy in Nursing in the College of Nursing at the University of Central Florida Orlando Florida Fall Term 2015 Major Professor: Karen Arioan © 2015 Kandis M. Natoli ii ABSTRACT The United States has established breastfeeding as an important health indicator within the Healthy People agenda. Healthy People target goals for breastfeeding initiation, duration, and exclusivity remain unmet. The US Surgeon General’s Office reports that lack of knowledge and widespread misinformation about breastfeeding are barriers to meeting Healthy People goals. Breastfeeding mothers are vulnerable to messages that cast doubt on their ability to breastfeed. Very little research has examined specific approaches to help people resist negative messages about health beliefs and behaviors. -

XI. COMPLICATIONS of PREGNANCY, Childbffith and the PUERPERIUM 630 Hydatidiform Mole Trophoblastic Disease NOS Vesicular Mole Ex
XI. COMPLICATIONS OF PREGNANCY, CHILDBffiTH AND THE PUERPERIUM PREGNANCY WITH ABORTIVE OUTCOME (630-639) 630 Hydatidiform mole Trophoblastic disease NOS Vesicular mole Excludes: chorionepithelioma (181) 631 Other abnormal product of conception Blighted ovum Mole: NOS carneous fleshy Excludes: with mention of conditions in 630 (630) 632 Missed abortion Early fetal death with retention of dead fetus Retained products of conception, not following spontaneous or induced abortion or delivery Excludes: failed induced abortion (638) missed delivery (656.4) with abnormal product of conception (630, 631) 633 Ectopic pregnancy Includes: ruptured ectopic pregnancy 633.0 Abdominal pregnancy 633.1 Tubalpregnancy Fallopian pregnancy Rupture of (fallopian) tube due to pregnancy Tubal abortion 633.2 Ovarian pregnancy 633.8 Other ectopic pregnancy Pregnancy: Pregnancy: cervical intraligamentous combined mesometric cornual mural - 355- 356 TABULAR LIST 633.9 Unspecified The following fourth-digit subdivisions are for use with categories 634-638: .0 Complicated by genital tract and pelvic infection [any condition listed in 639.0] .1 Complicated by delayed or excessive haemorrhage [any condition listed in 639.1] .2 Complicated by damage to pelvic organs and tissues [any condi- tion listed in 639.2] .3 Complicated by renal failure [any condition listed in 639.3] .4 Complicated by metabolic disorder [any condition listed in 639.4] .5 Complicated by shock [any condition listed in 639.5] .6 Complicated by embolism [any condition listed in 639.6] .7 With other