Correct Name for the Indian Flying Fox (Pteropodidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Daytime Behaviour of the Grey-Headed Flying Fox Pteropus Poliocephalus Temminck (Pteropodidae: Megachiroptera) at an Autumn/Winter Roost
DAYTIME BEHAVIOUR OF THE GREY-HEADED FLYING FOX PTEROPUS POLIOCEPHALUS TEMMINCK (PTEROPODIDAE: MEGACHIROPTERA) AT AN AUTUMN/WINTER ROOST K.A. CONNELL, U. MUNRO AND F.R. TORPY Connell KA, Munro U and Torpy FR, 2006. Daytime behaviour of the grey-headed flying fox Pteropus poliocephalus Temminck (Pteropodidae: Megachiroptera) at an autumn/winter roost. Australian Mammalogy 28: 7-14. The grey-headed flying fox (Pteropus poliocephalus Temminck) is a threatened large fruit bat endemic to Australia. It roosts in large colonies in rainforest patches, mangroves, open forest, riparian woodland and, as native habitat is reduced, increasingly in vegetation within urban environments. The general biology, ecology and behaviour of this bat remain largely unknown, which makes it difficult to effectively monitor, protect and manage this species. The current study provides baseline information on the daytime behaviour of P. poliocephalus in an autumn/winter roost in urban Sydney, Australia, between April and August 2003. The most common daytime behaviours expressed by the flying foxes were sleeping (most common), grooming, mating/courtship, and wing spreading (least common). Behaviours differed significantly between times of day and seasons (autumn and winter). Active behaviours (i.e., grooming, mating/courtship, wing spreading) occurred mainly in the morning, while sleeping predominated in the afternoon. Mating/courtship and wing spreading were significantly higher in April (reproductive period) than in winter (non-reproductive period). Grooming was the only behaviour that showed no significant variation between sample periods. These results provide important baseline data for future comparative studies on the behaviours of flying foxes from urban and ‘natural’ camps, and the development of management strategies for this species. -
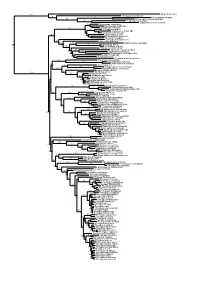
Figs1 ML Tree.Pdf
100 Megaderma lyra Rhinopoma hardwickei 71 100 Rhinolophus creaghi 100 Rhinolophus ferrumequinum 100 Hipposideros armiger Hipposideros commersoni 99 Megaerops ecaudatus 85 Megaerops niphanae 100 Megaerops kusnotoi 100 Cynopterus sphinx 98 Cynopterus horsfieldii 69 Cynopterus brachyotis 94 50 Ptenochirus minor 86 Ptenochirus wetmorei Ptenochirus jagori Dyacopterus spadiceus 99 Sphaerias blanfordi 99 97 Balionycteris maculata 100 Aethalops alecto 99 Aethalops aequalis Thoopterus nigrescens 97 Alionycteris paucidentata 33 99 Haplonycteris fischeri 29 Otopteropus cartilagonodus Latidens salimalii 43 88 Penthetor lucasi Chironax melanocephalus 90 Syconycteris australis 100 Macroglossus minimus 34 Macroglossus sobrinus 92 Boneia bidens 100 Harpyionycteris whiteheadi 69 Harpyionycteris celebensis Aproteles bulmerae 51 Dobsonia minor 100 100 80 Dobsonia inermis Dobsonia praedatrix 99 96 14 Dobsonia viridis Dobsonia peronii 47 Dobsonia pannietensis 56 Dobsonia moluccensis 29 Dobsonia anderseni 100 Scotonycteris zenkeri 100 Casinycteris ophiodon 87 Casinycteris campomaanensis Casinycteris argynnis 99 100 Eonycteris spelaea 100 Eonycteris major Eonycteris robusta 100 100 Rousettus amplexicaudatus 94 Rousettus spinalatus 99 Rousettus leschenaultii 100 Rousettus aegyptiacus 77 Rousettus madagascariensis 87 Rousettus obliviosus Stenonycteris lanosus 100 Megaloglossus woermanni 100 91 Megaloglossus azagnyi 22 Myonycteris angolensis 100 87 Myonycteris torquata 61 Myonycteris brachycephala 33 41 Myonycteris leptodon Myonycteris relicta 68 Plerotes anchietae -

Bat Count 2003
BAT COUNT 2003 Working to promote the long term, sustainable conservation of globally threatened flying foxes in the Philippines, by developing baseline population information, increasing public awareness, and training students and protected area managers in field monitoring techniques. 1 A Terminal Report Submitted by Tammy Mildenstein1, Apolinario B. Cariño2, and Samuel Stier1 1Fish and Wildlife Biology, University of Montana, USA 2Silliman University and Mt. Talinis – Twin Lakes Federation of People’s Organizations, Diputado Extension, Sibulan, Negros Oriental, Philippines Photo by: Juan Pablo Moreiras 2 EXECUTIVE SUMMARY Large flying foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to deforestation, unregulated hunting, and little conservation commitment from local governments. Despite the fact they are globally endangered and play essential ecological roles in forest regeneration as seed dispersers and pollinators, there have been only a few studies on these bats that provide information useful to their conservation management. Our project aims to promote the conservation of large flying foxes in the Philippines by providing protected area managers with the training and the baseline information necessary to design and implement a long-term management plan for flying foxes. We focused our efforts on the globally endangered Philippine endemics, Acerodon jubatus and Acerodon leucotis, and the bats that commonly roost with them, Pteropus hypomelanus, P. vampyrus lanensis, and P. pumilus which are thought to be declining in the Philippines. Local participation is an integral part of our project. We conducted the first national training workshop on flying fox population counts and conservation at the Subic Bay area. -

The Philippine Flying Foxes, Acerodon Jubatus and Pteropus Vampyrus Lanensis
Journal of Mammalogy, 86(4):719- 728, 2005 DIETARY HABITS OF THE WORLD’S LARGEST BATS: THE PHILIPPINE FLYING FOXES, ACERODON JUBATUS AND PTEROPUS VAMPYRUS LANENSIS Sam C. Stier* and Tammy L. M ildenstein College of Forestry and Conservation, University of Montana, Missoula, MT 59802, USA The endemic and endangered golden- crowned flying fox (Acerodon jubatus) coroosts with the much more common and widespread giant Philippine fmit bat (Pteropus vampyrus ianensis) in lowland dipterocarp forests throughout the Philippine Islands. The number of these mixed roost- colonies and the populations of flying foxes in them have declined dramatically in the last century. We used fecal analysis, interviews of bat hunters, and personal observations to describe the dietary habits of both bat species at one of the largest mixed roosts remaining, near Subic Bay, west- central Luzon. Dietary items were deemed “important” if used consistently on a seasonal basis or throughout the year, ubiquitously throughout the population, and if they were of clear nutritional value. Of the 771 droppings examined over a 2.5 -year period (1998-2000), seeds from Ficus were predominant in the droppings of both species and met these criteria, particularly hemiepiphytic species (41% of droppings of A. jubatus) and Ficus variegata (34% of droppings of P. v. ianensis and 22% of droppings of A. jubatus). Information from bat hunter interviews expanded our knowledge of the dietary habits of both bat species, and corroborated the fecal analyses and personal observations. Results from this study suggest that A. jubatus is a forest obligate, foraging on fruits and leaves from plant species restricted to lowland, mature natural forests, particularly using a small subset of hemiepiphytic and other Ficus species throughout the year. -

Emergence and Returning Activity in the Indian Flying Fox, Pteropus Giganteus (Chiroptera: Pteropodidae)
International Journal of Geography and Geology 1(1):1-9 International Journal of Geography and Geology journal homepage: http://aessweb.com/journal-detail.php?id=5011 EMERGENCE AND RETURNING ACTIVITY IN THE INDIAN FLYING FOX, PTEROPUS GIGANTEUS (CHIROPTERA: PTEROPODIDAE) M. R. Sudhakaran1 D. P. Swamidoss2 P. Parvathiraj3 ABSTRACT Approach: After a diurnal resting in the roost, bats adapts some behavioural pattern to get themselves active towards foraging there by involving in various activities. Their activity pattern differs from time to time depending on the change in climatic factors. But the behavioural activities they involved varies from time to time. Observation was done on the emergence and returning gate i. e the emergence or returning of first to last bat, pre emergence behaviour and post return behaviour, and influence of moonlight on foraging activity. Key Words: Post return activity, emergence gate, behaviour, pre-emergence behaviour. INTRODUCTION Most of the megachiropteran’s found in the sub tropics roost in tents, in manmade structures like old houses and temples and in foliages or hollows in trees, mainly to evade from the climatic factors, which affect them. Indian flying fox, Pteropus giganteus roosts in open foliages of trees, a peculiar character of this genus. Papers dealing with behavioural aspects of P. giganteus are scarce. Apart from Neuweiler (1969), other works on behavioural aspects focused on copulatory behaviour (Koilraj et al., 2001), roost preference (Acharya, 1936), mating (Bhatt, 1942), local migration (Breadow, 1931; Nelson, 1965a) and general ecology and biology (Brosset, 1962). Bats roosting in closed environments involved in various behavioural activities during their emergence (Kunz, 1982) like light sampling, flying inside its roost etc. -

A Review of the Pteropus Rufus (É. Geoffroy, 1803) Colonies Within The
ARTICLE IN PRESS - EARLY VIEW MADAGASCAR CONSERVATION & DEVELOPMENT VOLUME 11 | ISSUE 1 — JUNE 2016 page 1 ARTICLE http://dx.doi.org/10.4314/mcd.v11i1.7 A review of the Pteropus rufus (É. Geoffroy, 1803) colonies within the Tolagnaro region of southeast Madagascar – an assessment of neoteric threats and conservation condition Sam Hyde RobertsI, Mark D. JacobsI, Ryan M. ClarkI, Correspondence: Charlotte M. DalyII, Longosoa H. TsimijalyIII, Retsiraiky J. Sam Hyde Roberts RossizelaIII, Samuel T. PrettymanI SEED Madagascar, Studio 7, 1A Beethoven Street, London W10 4LG, United Kingdom Email: [email protected] ABSTRACT soit parce qu’elles se sont déplacées suite à des dérangements. We surveyed 10 Pteropus rufus roost sites within the sou- Les effectifs d’une seule colonie semblent avoir diminué de theastern Anosy Region of Madagascar to provide an update on manière significative tandis que ceux de trois autres colonies the areas’ known flying fox population and its conservation status. semblent avoir été maintenus à leur niveau. Notre étude a montré We report on two new colonies from Manambaro and Mandena que l'abondance globale de P. rufus dans la région n’a augmenté and provide an account of the colonies first reported and last as- que d’un pourcent depuis 2006 et que cette augmentation était le sessed in 2006. Currently only a solitary roost site receives any résultat de la protection garantie au dortoir dans la réserve privée formal protection (Berenty) whereas further two colonies rely so- de Berenty. À la lumière d'un décret qui a imposé une période de lely on taboo ‘fady’ for their security. -

Population Fluctuation at Indian Flying Fox (Pteropus Giganteus) Colonies
Available online at www.ijpab.com ISSN: 2320 – 7051 Int. J. Pure App. Biosci. 2 (4): 184-188 (2014) Research Article INTERNATIONAL JO URNAL OF PURE & APPLIED BIOSCIENCE Population fluctuation at Indian Flying Fox (Pteropus giganteus ) colonies in the Kacharighat Roosting Site of Dhubri district of Assam Monika Khatun¹, Azad Ali²* and Santanu Sarma³ ¹Researcher, Bat Research and Conservation Division (BRCD); Biodiversity and Ecological Research Centre (BERC); Department of Zoology, B. N. College, Dhubri-783324, Assam, India ²“Bat Research and Conservation Division (BRCD)”; Coordinator, “Biodiversity and Ecological Research Centre (BERC)”; Assistant Professor and Head, Department of Zoology, B.N. College, Dhubri-783324, Assam, India ³Assistant Professor, Department of Zoology, B. N. College, Dhubri, Assam *Corresponding Author E-mail: [email protected] ABSTRACT The Indian Flying Fox (Pteropus giganteus) is a species of flying fox of the Pteropodidae family under the suborder Megachiroptera. It is one of the largest fruit bat species found in the Indian subcontinent. They have one to two young. This bat is gregarious and roosts in colonies which can number up to 1,000 individuals, during the day. Due to various anthropogenic and environmental causes their populations are declining alarmingly. Current study has been aimed to know the population and its fluctuation in the “Kacharighat Roosting Site” of Dhubri town area of Assam. “Direct roost count” method was followed to estimate the population size of the colony. Observations were mostly done with the naked eyes . All total four roosting spots were identified in the roosting site. Highest population was recorded at 775 in the site. Total population of the roosting site was ranged between 720 and 775 during the study. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

A Checklist of the Mammals of South-East Asia
A Checklist of the Mammals of South-east Asia A Checklist of the Mammals of South-east Asia PHOLIDOTA Pangolin (Manidae) 1 Sunda Pangolin (Manis javanica) 2 Chinese Pangolin (Manis pentadactyla) INSECTIVORA Gymnures (Erinaceidae) 3 Moonrat (Echinosorex gymnurus) 4 Short-tailed Gymnure (Hylomys suillus) 5 Chinese Gymnure (Hylomys sinensis) 6 Large-eared Gymnure (Hylomys megalotis) Moles (Talpidae) 7 Slender Shrew-mole (Uropsilus gracilis) 8 Kloss's Mole (Euroscaptor klossi) 9 Large Chinese Mole (Euroscaptor grandis) 10 Long-nosed Chinese Mole (Euroscaptor longirostris) 11 Small-toothed Mole (Euroscaptor parvidens) 12 Blyth's Mole (Parascaptor leucura) 13 Long-tailed Mole (Scaptonyx fuscicauda) Shrews (Soricidae) 14 Lesser Stripe-backed Shrew (Sorex bedfordiae) 15 Myanmar Short-tailed Shrew (Blarinella wardi) 16 Indochinese Short-tailed Shrew (Blarinella griselda) 17 Hodgson's Brown-toothed Shrew (Episoriculus caudatus) 18 Bailey's Brown-toothed Shrew (Episoriculus baileyi) 19 Long-taied Brown-toothed Shrew (Episoriculus macrurus) 20 Lowe's Brown-toothed Shrew (Chodsigoa parca) 21 Van Sung's Shrew (Chodsigoa caovansunga) 22 Mole Shrew (Anourosorex squamipes) 23 Himalayan Water Shrew (Chimarrogale himalayica) 24 Styan's Water Shrew (Chimarrogale styani) Page 1 of 17 Database: Gehan de Silva Wijeyeratne, www.jetwingeco.com A Checklist of the Mammals of South-east Asia 25 Malayan Water Shrew (Chimarrogale hantu) 26 Web-footed Water Shrew (Nectogale elegans) 27 House Shrew (Suncus murinus) 28 Pygmy White-toothed Shrew (Suncus etruscus) 29 South-east -

Babesial Infection in the Madagascan Flying Fox, Pteropus Rufus É
Ranaivoson et al. Parasites & Vectors (2019) 12:51 https://doi.org/10.1186/s13071-019-3300-7 RESEARCH Open Access Babesial infection in the Madagascan flying fox, Pteropus rufus É. Geoffroy, 1803 Hafaliana C. Ranaivoson1,2, Jean-Michel Héraud1, Heidi K. Goethert3, Sam R. Telford III3, Lydia Rabetafika2† and Cara E. Brook4,5*† Abstract Background: Babesiae are erythrocytic protozoans, which infect the red blood cells of vertebrate hosts to cause disease. Previous studies have described potentially pathogenic infections of Babesia vesperuginis in insectivorous bats in Europe, the Americas and Asia. To date, no babesial infections have been documented in the bats of Madagascar, or in any frugivorous bat species worldwide. Results: We used standard microscopy and conventional PCR to identify babesiae in blood from the endemic Madagascan flying fox (Pteropus rufus). Out of 203 P. rufus individuals captured between November 2013 and January 2016 and screened for erythrocytic parasites, nine adult males (4.43%) were infected with babesiae. Phylogenetic analysis of sequences obtained from positive samples indicates that they cluster in the Babesia microti clade, which typically infect felids, rodents, primates, and canids, but are distinct from B. vesperuginis previously described in bats. Statistical analysis of ecological trends in the data suggests that infections were most commonly observed in the rainy season and in older-age individuals. No pathological effects of infection on the host were documented; age-prevalence patterns indicated susceptible-infectious (SI) transmission dynamics characteristic of a non-immunizing persistent infection. Conclusions: To our knowledge, this study is the first report of any erythrocytic protozoan infecting Madagascan fruit bats and the first record of a babesial infection in a pteropodid fruit bat globally. -

Flying Foxes): Preliminary Chemical Comparisons Among Species Jamie Wagner SIT Study Abroad
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by World Learning SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Fall 2008 Glandular Secretions of Male Pteropus (Flying Foxes): Preliminary Chemical Comparisons Among Species Jamie Wagner SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Animal Sciences Commons, and the Biology Commons Recommended Citation Wagner, Jamie, "Glandular Secretions of Male Pteropus (Flying Foxes): Preliminary Chemical Comparisons Among Species" (2008). Independent Study Project (ISP) Collection. 559. https://digitalcollections.sit.edu/isp_collection/559 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. Glandular secretions of male Pteropus (flying foxes): Preliminary chemical comparisons among species By Jamie Wagner Academic Director: Tony Cummings Project Advisor: Dr. Hugh Spencer Oberlin College Biology and Neuroscience Cape Tribulation, Australia Submitted in partial fulfillment of the requirements for Australia: Natural and Cultural Ecology, SIT Study Abroad, Fall 2008 1 1. Abstract Chemosignaling – passing information by means of chemical compounds that can be detected by members of the same species – is a very important form of communication for most mammals. Flying fox males have odiferous marking secretions on their neck-ruffs that include a combination of secretion from the neck gland and from the urogenital tract; males use this substance to establish territory, especially during the mating season. -

Habitat Selection of Endangered and Endemic Large Flying-Foxes in Subic
Biological Conservation 126 (2005) 93–102 www.elsevier.com/locate/biocon Habitat selection of endangered and endemic large Xying-foxes in Subic Bay, Philippines Tammy L. Mildenstein a,¤, Sam C. Stier a, C.E. Nuevo-Diego b, L. Scott Mills c a c/o US Peace Corps, Patio Madrigal Compound, 2775 Roxas Blvd., Pasay City, Metro Manila, Philippines b 9872 Isarog St. Umali Subdivision, Los Baños, Laguna 4031, Philippines c Wildlife Biology Program, School of Forestry, University of Montana, Missoula, MT 59812-0596, USA Received 6 June 2004 Available online 5 July 2005 Abstract Large Xying-foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to high levels of deforestation and hunting and eVectively little local conservation commitment. The forest at Subic Bay, Philippines, supports a rare, large colony of vulnerable Philippine giant fruit bats (Pteropus vampyrus lanensis) and endangered and endemic golden-crowned Xying-foxes (Acerodon jubatus). These large Xying-foxes are optimal for conservation focus, because in addition to being keystone, Xagship, and umbrella species, the bats are important to Subic Bay’s economy and its indigenous cultures. Habitat selection information stream- lines management’s eVorts to protect and conserve these popular but threatened animals. We used radio telemetry to describe the bats’ nighttime use of habitat on two ecological scales: vegetation and microhabitat. The fruit bats used the entire 14,000 ha study area, including all of Subic Bay Watershed Reserve, as well as neighboring forests just outside the protected area boundaries. Their recorded foraging locations ranged between 0.4 and 12 km from the roost.