Parturition in Geoffroy's Rousette Fruit Bat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Daytime Behaviour of the Grey-Headed Flying Fox Pteropus Poliocephalus Temminck (Pteropodidae: Megachiroptera) at an Autumn/Winter Roost
DAYTIME BEHAVIOUR OF THE GREY-HEADED FLYING FOX PTEROPUS POLIOCEPHALUS TEMMINCK (PTEROPODIDAE: MEGACHIROPTERA) AT AN AUTUMN/WINTER ROOST K.A. CONNELL, U. MUNRO AND F.R. TORPY Connell KA, Munro U and Torpy FR, 2006. Daytime behaviour of the grey-headed flying fox Pteropus poliocephalus Temminck (Pteropodidae: Megachiroptera) at an autumn/winter roost. Australian Mammalogy 28: 7-14. The grey-headed flying fox (Pteropus poliocephalus Temminck) is a threatened large fruit bat endemic to Australia. It roosts in large colonies in rainforest patches, mangroves, open forest, riparian woodland and, as native habitat is reduced, increasingly in vegetation within urban environments. The general biology, ecology and behaviour of this bat remain largely unknown, which makes it difficult to effectively monitor, protect and manage this species. The current study provides baseline information on the daytime behaviour of P. poliocephalus in an autumn/winter roost in urban Sydney, Australia, between April and August 2003. The most common daytime behaviours expressed by the flying foxes were sleeping (most common), grooming, mating/courtship, and wing spreading (least common). Behaviours differed significantly between times of day and seasons (autumn and winter). Active behaviours (i.e., grooming, mating/courtship, wing spreading) occurred mainly in the morning, while sleeping predominated in the afternoon. Mating/courtship and wing spreading were significantly higher in April (reproductive period) than in winter (non-reproductive period). Grooming was the only behaviour that showed no significant variation between sample periods. These results provide important baseline data for future comparative studies on the behaviours of flying foxes from urban and ‘natural’ camps, and the development of management strategies for this species. -
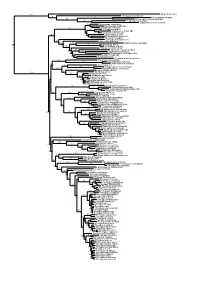
Figs1 ML Tree.Pdf
100 Megaderma lyra Rhinopoma hardwickei 71 100 Rhinolophus creaghi 100 Rhinolophus ferrumequinum 100 Hipposideros armiger Hipposideros commersoni 99 Megaerops ecaudatus 85 Megaerops niphanae 100 Megaerops kusnotoi 100 Cynopterus sphinx 98 Cynopterus horsfieldii 69 Cynopterus brachyotis 94 50 Ptenochirus minor 86 Ptenochirus wetmorei Ptenochirus jagori Dyacopterus spadiceus 99 Sphaerias blanfordi 99 97 Balionycteris maculata 100 Aethalops alecto 99 Aethalops aequalis Thoopterus nigrescens 97 Alionycteris paucidentata 33 99 Haplonycteris fischeri 29 Otopteropus cartilagonodus Latidens salimalii 43 88 Penthetor lucasi Chironax melanocephalus 90 Syconycteris australis 100 Macroglossus minimus 34 Macroglossus sobrinus 92 Boneia bidens 100 Harpyionycteris whiteheadi 69 Harpyionycteris celebensis Aproteles bulmerae 51 Dobsonia minor 100 100 80 Dobsonia inermis Dobsonia praedatrix 99 96 14 Dobsonia viridis Dobsonia peronii 47 Dobsonia pannietensis 56 Dobsonia moluccensis 29 Dobsonia anderseni 100 Scotonycteris zenkeri 100 Casinycteris ophiodon 87 Casinycteris campomaanensis Casinycteris argynnis 99 100 Eonycteris spelaea 100 Eonycteris major Eonycteris robusta 100 100 Rousettus amplexicaudatus 94 Rousettus spinalatus 99 Rousettus leschenaultii 100 Rousettus aegyptiacus 77 Rousettus madagascariensis 87 Rousettus obliviosus Stenonycteris lanosus 100 Megaloglossus woermanni 100 91 Megaloglossus azagnyi 22 Myonycteris angolensis 100 87 Myonycteris torquata 61 Myonycteris brachycephala 33 41 Myonycteris leptodon Myonycteris relicta 68 Plerotes anchietae -

Bat Count 2003
BAT COUNT 2003 Working to promote the long term, sustainable conservation of globally threatened flying foxes in the Philippines, by developing baseline population information, increasing public awareness, and training students and protected area managers in field monitoring techniques. 1 A Terminal Report Submitted by Tammy Mildenstein1, Apolinario B. Cariño2, and Samuel Stier1 1Fish and Wildlife Biology, University of Montana, USA 2Silliman University and Mt. Talinis – Twin Lakes Federation of People’s Organizations, Diputado Extension, Sibulan, Negros Oriental, Philippines Photo by: Juan Pablo Moreiras 2 EXECUTIVE SUMMARY Large flying foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to deforestation, unregulated hunting, and little conservation commitment from local governments. Despite the fact they are globally endangered and play essential ecological roles in forest regeneration as seed dispersers and pollinators, there have been only a few studies on these bats that provide information useful to their conservation management. Our project aims to promote the conservation of large flying foxes in the Philippines by providing protected area managers with the training and the baseline information necessary to design and implement a long-term management plan for flying foxes. We focused our efforts on the globally endangered Philippine endemics, Acerodon jubatus and Acerodon leucotis, and the bats that commonly roost with them, Pteropus hypomelanus, P. vampyrus lanensis, and P. pumilus which are thought to be declining in the Philippines. Local participation is an integral part of our project. We conducted the first national training workshop on flying fox population counts and conservation at the Subic Bay area. -

Attacked from Above and Below, New Observations of Cooperative and Solitary Predators on Roosting Cave Bats
bioRxiv preprint doi: https://doi.org/10.1101/550582; this version posted February 17, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Attacked from above and below, new observations of cooperative and solitary predators on roosting cave bats Krizler Cejuela. Tanalgo1, 2, 3, 7*, Dave L. Waldien4, 5, 6, Norma Monfort6, Alice Catherine Hughes1, 3* 1Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 2International College, University of Chinese Academy of Sciences, Beijing 100049, People’s Republic of China 3Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 4Harrison Institute, Bowerwood House, 15 St Botolph’s Road, Sevenoaks, Kent, TN13 3AQ, United Kingdom 5Christopher Newport University, 1 Avenue of the Arts, Newport News, VA 23606, United States of America 6Philippine Bats for Peace Foundation Inc., 5 Ramona Townhomes, Guadalupe Village, Lanang, Davao City 7Department of Biological Sciences, College of Arts and Sciences, University of Southern Mindanao, Kabacan 9407, North Cotabato, the Republic of the Philippines Corresponding authors: ACH ([email protected]) and KCT ([email protected]) Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China Abstract Predation of bats in their roosts has previously only been attributed to a limited number of species such as various raptors, owls, and snakes. -

The Philippine Flying Foxes, Acerodon Jubatus and Pteropus Vampyrus Lanensis
Journal of Mammalogy, 86(4):719- 728, 2005 DIETARY HABITS OF THE WORLD’S LARGEST BATS: THE PHILIPPINE FLYING FOXES, ACERODON JUBATUS AND PTEROPUS VAMPYRUS LANENSIS Sam C. Stier* and Tammy L. M ildenstein College of Forestry and Conservation, University of Montana, Missoula, MT 59802, USA The endemic and endangered golden- crowned flying fox (Acerodon jubatus) coroosts with the much more common and widespread giant Philippine fmit bat (Pteropus vampyrus ianensis) in lowland dipterocarp forests throughout the Philippine Islands. The number of these mixed roost- colonies and the populations of flying foxes in them have declined dramatically in the last century. We used fecal analysis, interviews of bat hunters, and personal observations to describe the dietary habits of both bat species at one of the largest mixed roosts remaining, near Subic Bay, west- central Luzon. Dietary items were deemed “important” if used consistently on a seasonal basis or throughout the year, ubiquitously throughout the population, and if they were of clear nutritional value. Of the 771 droppings examined over a 2.5 -year period (1998-2000), seeds from Ficus were predominant in the droppings of both species and met these criteria, particularly hemiepiphytic species (41% of droppings of A. jubatus) and Ficus variegata (34% of droppings of P. v. ianensis and 22% of droppings of A. jubatus). Information from bat hunter interviews expanded our knowledge of the dietary habits of both bat species, and corroborated the fecal analyses and personal observations. Results from this study suggest that A. jubatus is a forest obligate, foraging on fruits and leaves from plant species restricted to lowland, mature natural forests, particularly using a small subset of hemiepiphytic and other Ficus species throughout the year. -

A Review of the Pteropus Rufus (É. Geoffroy, 1803) Colonies Within The
ARTICLE IN PRESS - EARLY VIEW MADAGASCAR CONSERVATION & DEVELOPMENT VOLUME 11 | ISSUE 1 — JUNE 2016 page 1 ARTICLE http://dx.doi.org/10.4314/mcd.v11i1.7 A review of the Pteropus rufus (É. Geoffroy, 1803) colonies within the Tolagnaro region of southeast Madagascar – an assessment of neoteric threats and conservation condition Sam Hyde RobertsI, Mark D. JacobsI, Ryan M. ClarkI, Correspondence: Charlotte M. DalyII, Longosoa H. TsimijalyIII, Retsiraiky J. Sam Hyde Roberts RossizelaIII, Samuel T. PrettymanI SEED Madagascar, Studio 7, 1A Beethoven Street, London W10 4LG, United Kingdom Email: [email protected] ABSTRACT soit parce qu’elles se sont déplacées suite à des dérangements. We surveyed 10 Pteropus rufus roost sites within the sou- Les effectifs d’une seule colonie semblent avoir diminué de theastern Anosy Region of Madagascar to provide an update on manière significative tandis que ceux de trois autres colonies the areas’ known flying fox population and its conservation status. semblent avoir été maintenus à leur niveau. Notre étude a montré We report on two new colonies from Manambaro and Mandena que l'abondance globale de P. rufus dans la région n’a augmenté and provide an account of the colonies first reported and last as- que d’un pourcent depuis 2006 et que cette augmentation était le sessed in 2006. Currently only a solitary roost site receives any résultat de la protection garantie au dortoir dans la réserve privée formal protection (Berenty) whereas further two colonies rely so- de Berenty. À la lumière d'un décret qui a imposé une période de lely on taboo ‘fady’ for their security. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Babesial Infection in the Madagascan Flying Fox, Pteropus Rufus É
Ranaivoson et al. Parasites & Vectors (2019) 12:51 https://doi.org/10.1186/s13071-019-3300-7 RESEARCH Open Access Babesial infection in the Madagascan flying fox, Pteropus rufus É. Geoffroy, 1803 Hafaliana C. Ranaivoson1,2, Jean-Michel Héraud1, Heidi K. Goethert3, Sam R. Telford III3, Lydia Rabetafika2† and Cara E. Brook4,5*† Abstract Background: Babesiae are erythrocytic protozoans, which infect the red blood cells of vertebrate hosts to cause disease. Previous studies have described potentially pathogenic infections of Babesia vesperuginis in insectivorous bats in Europe, the Americas and Asia. To date, no babesial infections have been documented in the bats of Madagascar, or in any frugivorous bat species worldwide. Results: We used standard microscopy and conventional PCR to identify babesiae in blood from the endemic Madagascan flying fox (Pteropus rufus). Out of 203 P. rufus individuals captured between November 2013 and January 2016 and screened for erythrocytic parasites, nine adult males (4.43%) were infected with babesiae. Phylogenetic analysis of sequences obtained from positive samples indicates that they cluster in the Babesia microti clade, which typically infect felids, rodents, primates, and canids, but are distinct from B. vesperuginis previously described in bats. Statistical analysis of ecological trends in the data suggests that infections were most commonly observed in the rainy season and in older-age individuals. No pathological effects of infection on the host were documented; age-prevalence patterns indicated susceptible-infectious (SI) transmission dynamics characteristic of a non-immunizing persistent infection. Conclusions: To our knowledge, this study is the first report of any erythrocytic protozoan infecting Madagascan fruit bats and the first record of a babesial infection in a pteropodid fruit bat globally. -

Investigating the Role of Bats in Emerging Zoonoses
12 ISSN 1810-1119 FAO ANIMAL PRODUCTION AND HEALTH manual INVESTIGATING THE ROLE OF BATS IN EMERGING ZOONOSES Balancing ecology, conservation and public health interest Cover photographs: Left: © Jon Epstein. EcoHealth Alliance Center: © Jon Epstein. EcoHealth Alliance Right: © Samuel Castro. Bureau of Animal Industry Philippines 12 FAO ANIMAL PRODUCTION AND HEALTH manual INVESTIGATING THE ROLE OF BATS IN EMERGING ZOONOSES Balancing ecology, conservation and public health interest Edited by Scott H. Newman, Hume Field, Jon Epstein and Carol de Jong FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2011 Recommended Citation Food and Agriculture Organisation of the United Nations. 2011. Investigating the role of bats in emerging zoonoses: Balancing ecology, conservation and public health interests. Edited by S.H. Newman, H.E. Field, C.E. de Jong and J.H. Epstein. FAO Animal Production and Health Manual No. 12. Rome. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. -

Flying Foxes): Preliminary Chemical Comparisons Among Species Jamie Wagner SIT Study Abroad
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by World Learning SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Fall 2008 Glandular Secretions of Male Pteropus (Flying Foxes): Preliminary Chemical Comparisons Among Species Jamie Wagner SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Animal Sciences Commons, and the Biology Commons Recommended Citation Wagner, Jamie, "Glandular Secretions of Male Pteropus (Flying Foxes): Preliminary Chemical Comparisons Among Species" (2008). Independent Study Project (ISP) Collection. 559. https://digitalcollections.sit.edu/isp_collection/559 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. Glandular secretions of male Pteropus (flying foxes): Preliminary chemical comparisons among species By Jamie Wagner Academic Director: Tony Cummings Project Advisor: Dr. Hugh Spencer Oberlin College Biology and Neuroscience Cape Tribulation, Australia Submitted in partial fulfillment of the requirements for Australia: Natural and Cultural Ecology, SIT Study Abroad, Fall 2008 1 1. Abstract Chemosignaling – passing information by means of chemical compounds that can be detected by members of the same species – is a very important form of communication for most mammals. Flying fox males have odiferous marking secretions on their neck-ruffs that include a combination of secretion from the neck gland and from the urogenital tract; males use this substance to establish territory, especially during the mating season. -

Taxonomy and Natural History of the Southeast Asian Fruit-Bat Genus Dyacopterus
Journal of Mammalogy, 88(2):302–318, 2007 TAXONOMY AND NATURAL HISTORY OF THE SOUTHEAST ASIAN FRUIT-BAT GENUS DYACOPTERUS KRISTOFER M. HELGEN,* DIETER KOCK,RAI KRISTIE SALVE C. GOMEZ,NINA R. INGLE, AND MARTUA H. SINAGA Division of Mammals, National Museum of Natural History (NHB 390, MRC 108), Smithsonian Institution, P.O. Box 37012, Washington, D.C. 20013-7012, USA (KMH) Forschungsinstitut Senckenberg, Senckenberganlage 25, Frankfurt, D-60325, Germany (DK) Philippine Eagle Foundation, VAL Learning Village, Ruby Street, Marfori Heights, Davao City, 8000, Philippines (RKSCG) Department of Natural Resources, Cornell University, Ithaca, NY 14853, USA (NRI) Division of Mammals, Field Museum of Natural History, Chicago, IL 60605, USA (NRI) Museum Zoologicum Bogoriense, Jl. Raya Cibinong Km 46, Cibinong 16911, Indonesia (MHS) The pteropodid genus Dyacopterus Andersen, 1912, comprises several medium-sized fruit-bat species endemic to forested areas of Sundaland and the Philippines. Specimens of Dyacopterus are sparsely represented in collections of world museums, which has hindered resolution of species limits within the genus. Based on our studies of most available museum material, we review the infrageneric taxonomy of Dyacopterus using craniometric and other comparisons. In the past, 2 species have been described—D. spadiceus (Thomas, 1890), described from Borneo and later recorded from the Malay Peninsula, and D. brooksi Thomas, 1920, described from Sumatra. These 2 nominal taxa are often recognized as species or conspecific subspecies representing these respective populations. Our examinations instead suggest that both previously described species of Dyacopterus co-occur on the Sunda Shelf—the smaller-skulled D. spadiceus in peninsular Malaysia, Sumatra, and Borneo, and the larger-skulled D. -

PARASITIC on MEGACHIROPTERAN BATS X
Pacific Insects Monograph 28: 213-243 20 June 1971 REVIEW OF THE STREBLIDAE (Diptera) PARASITIC ON MEGACHIROPTERAN BATS x By T. C. Maa2 Abstract. Of the 16 streblid species previously recorded as parasites of the Megachiroptera, only 6 are here considered to be correctly so associated. Five of these 6 species are re-assigned to a new genus and only 1 is retained in the genus Brachytarsina (^Nycteribosca). These 2 genera are each divided into 2 subgenera and their host relationships, distributional patterns and evolutionary trends are discussed. Earlier records of the species are critically reviewed and are incorporated with new data which are based on some 650 specimens. The new taxa described are Megastrebla, n. gen. (type N. gigantea Speiser); Aoroura, n. subgen, (type N. nigriceps Jobling); Psilacris, n. subgen, (type N. longiarista Jobling); M. (A) limbooliati, n. sp. (Malaya, Borneo); M. (M.) gigantea kaluzvawae, n. ssp. (Fergusson I.); M. (M) gigantea salomonis, n. ssp. (Solomon Is.); M. (M) parvior papuae, n. ssp. (New Guinea). Streblid batflies are rarely found on the suborder Megachiroptera, composed of the single family Pteropodidae, whose members are generally referred to as fruit bats. Only 16 species have been recorded on these bats. A closer examination of the pub lished records clearly indicates that 10 of these 16 species (see Appendix II) should not be considered true parasites of the Megachiroptera; available data support the con cept that no streblids normally breed simultaneously on both the Megachiroptera and Microchiroptera, and among the 39 genera of the former suborder, only those which usually roost in partially illuminated caves and rock-crevices serve as normal breeding hosts of Streblidae.