TOPIC 1. RADIANT ENERGY the Relationship Between the Sun And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Novel and Cost Effective Radiant Heat Flux Gauge This Paper Presents a Patent-Pending Methodology of Measuring Radiation Emiss
A Novel and Cost Effective Radiant Heat Flux Gauge S. Safaei and A. S. Rangwala Department of Fire Protection Engineering, Worcester Polytechnic Institution, Worcester, MA, 01609, USA V. Raghavan Department of Mechanical Engineering Indian Institute of Technology Madras, Chennai, India T.M. Muruganandam, Department of Aerospace Engineering, National Center for Combustion Research and Development, Indian Institute of Technology Madras, Chennai, India This paper presents a patent-pending methodology of measuring radiation emissions from fires, for the purposes of more practical optical flame detection and analysis. Flame radiation comprises of three phenomena: chemiluminescence, photoluminescence, and thermal radiation. The first two types are caused by elementary breakdown reactions of the reactants and the molecular excitations of products such as H2O and CO2, respectively. These emissions are within narrow bands of electromagnetic radiation wavelengths, and due to their specific molecular physics, are largely fuel dependant. The third type, thermal radiation emissions come from high temperature soot, which is a blanket term for any carbonaceous intermittent species of the combustion reaction. The amount and temperature of soot is case-specific, as it is influenced by fuel type, geometries, fuel-oxidizer premixing and the resulting level of combustion efficiency. However since naturally occurring fire hazards are diffusion flames, soot is present in high quantities and the majority of the radiation can be attributed to soot alone [1 – 2]. These soot emissions are over large ranges of the electromagnetic spectrum, from ultraviolet all the way to Long wave Infrared (LWIR), following the classic Planck Law for Blackbody emitters. This distribution of radiant energy, E, is highly dependent on the temperature of the emitter, T, due to its exponential relation E=T4. -

Low Cost Solar Thermoelectric Water Floating Device to Supply Measurement Platform
78 IAPGOŚ 4/2019 p-ISSN 2083-0157, e-ISSN 2391-6761 DOI: 10.35784/IAPGOS.734 LOW COST SOLAR THERMOELECTRIC WATER FLOATING DEVICE TO SUPPLY MEASUREMENT PLATFORM Andrzej Nowrot1, Monika Mikołajczyk2, Anna Manowska1, Joachim Pielot1, Antoni Wojaczek1 1Silesian University of Technology, Department of Electrical Engineering and Automation in Industry, Gliwice, Poland, 2Famur Institute Ltd, Katowice, Poland Abstract. This work presents the prototype of the solar – thermoelectric device, which can float on water surface. It produces electrical energy as a result of the Seebeck effect in a commercial, low-cost Peltier module. The main application of the device will be an autonomous and a floating measurement platform. An important advantage of the presented solution is the possibility to work alike at day, when a solar light heats the surface of the absorber, and at night, when the different of temperatures between air and water causes the heat flux and in an effect the electricity. The device is capable of working for many cloudy days and also in winter on very short days. The presented device is based on low-cost and widely available components. Keywords: thermoelectric devices, solar power generation, energy conversion TERMOELEKTRYCZNE URZĄDZENIE SOLARNE DO ZASILANIA PLATFORMY POMIAROWEJ Streszczenie. W pracy zaprezentowano prototypowe solarne urządzenie termoelektryczne pływające po powierzchni wody. Wytwarza ono w niedrogim, komercyjnym module Peltiera energię elektryczną w wyniku zachodzącego w nim zjawiska Seebecka. Docelowo głównym obszarem aplikacyjnym urządzenia będzie zasilanie autonomicznej, pływającej platformy pomiarowa do monitorowania parametrów środowiskowych. Istotną zaletą przedstawionego rozwiązania jest możliwość pracy zarówno w dzień, gdy światło słoneczne ogrzewa powierzchnię absorbera, jak również w nocy, gdy różnica temperatur między powietrzem a wodą powoduje powstawanie strumienia ciepła w module Peltiera. -

United States Patent [191 4,2313J97 Sher
United States Patent [191 4,2313J97 Sher [54] RADIANT ENERGY TO ELECTRIC ENERGY 4,084,101 4/1978 Sher ..................................... 290/1 R CONVERTER 4,096,393 6/1978 Sher ..................................... 290A R [76] Inventor: Arden Sher, 108 Charles River OTHER PUBLICATIONS Landing Rd., Williamsburg, Va. R. Solomon et al., “Polarization in LaF,”, J. Appl. Phys., 23185 VO~.37, pp. 3427-3432, (1966). [ * 3 Notice: The portion of the term of this patent A. Sher et al., “Transport Properties of LaF3”, Phys. subsequent to Apr. 11,1995, has been Rev., vol. 144, pp. 593-604, (1966). disclaimed. J. E. Drummond, “Electrical Power Conversion”, IECEC Record ’75, pp. 569-575. [21] Appl. No.: 889,514 S. B. Skinner, “Thermodielectric Energy Conversion [22] Filed: Mar. 23,1978 by Thin Films: Experiment & Theory”, Intersociety Energy Conv. Eng. Con$, (1967), pp. 865-873. Related U.S. Application Data A. Sher et al., “LaF3 Infrared Detector”, Appl. Phys. Lett., vol. 28, pp. 676-678, (1976). [63] Continuation-in-part of Ser. No. 631,689, Nov. 13, 1975, Pat. No. 4,084,101. Primary Examiner-Aaron Weisstuch Attorney, Agent, or Firm-Lowe, King, Price and [51] Int. Cl.2 ............................................. HQlL31/Q4 Becker [52] U.S. Cl. ............................... 136/89 SP; 290/1 R 361/280; 361/282; 310/308; 310/309; 250/211 1571 ABSTRACT 250/212; 250/336 R; Radiant energy is converted into electric energy by [58] Field of Search ............ 136/89 R, 89 NB, 89 SP; irradiating a capacitor including an ionic dielectric. The 250/211 R, 212, 336; 290/1 R 361/280,282; 310/308, 309 dielectric is a sintered crystal superionic conductor, e.g., lanthanum trifluoride, lanthanum trichloride, or [561 References Cited silver bromide, so that a multiplicity of crystallites exist U.S. -

LESSON 1 What Is Energy?
LESSON 1 What is Energy? Overview In this lesson students review what they know about energy in everyday life, define it, learn the different energy forms through play, and differentiate between potential and kinetic energy. Student • I can use my own words to show I understand “energy.” Learning • I can describe the main forms of energy and give examples. Targets • I can show potential and kinetic energy with my body and I can give examples of each. • I can give examples of how I use energy every day. NGSS MS-PS3-5. Construct, use, and present arguments to support the claim that when the kinetic energy of an object changes, energy is transferred to or from the object. [Clarification Statement: Examples of empirical evidence used in arguments could include an inventory or other representation of the energy before and after the transfer in the form of temperature changes or motion of object.] [Assessment Boundary: Assessment does not include calculations of energy.] Background Energy is part of everything that happens in the world and everything we do. Energy can be in the form of heat, light, gravity, sound, motion, chemical reactions, or electricity. Energy is the ability to do work or make a change. Nine forms of energy: (see Overhead 1 “Forms of Energy”) • Chemical: Chemical energy is the energy stored in the bonds of atoms and molecules. Biomass, petroleum, natural gas, propane and coal are examples of stored chemical energy. • Nuclear: Nuclear energy is the energy stored in the nucleus of an atom. It is the energy that holds the nucleus together. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -
Properties of Electromagnetic Waves Any Electromagnetic Wave Must Satisfy Four Basic Conditions: 1
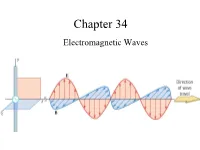
Chapter 34 Electromagnetic Waves The Goal of the Entire Course Maxwell’s Equations: Maxwell’s Equations James Clerk Maxwell •1831 – 1879 •Scottish theoretical physicist •Developed the electromagnetic theory of light •His successful interpretation of the electromagnetic field resulted in the field equations that bear his name. •Also developed and explained – Kinetic theory of gases – Nature of Saturn’s rings – Color vision Start at 12:50 https://www.learner.org/vod/vod_window.html?pid=604 Correcting Ampere’s Law Two surfaces S1 and S2 near the plate of a capacitor are bounded by the same path P. Ampere’s Law states that But it is zero on S2 since there is no conduction current through it. This is a contradiction. Maxwell fixed it by introducing the displacement current: Fig. 34-1, p. 984 Maxwell hypothesized that a changing electric field creates an induced magnetic field. Induced Fields . An increasing solenoid current causes an increasing magnetic field, which induces a circular electric field. An increasing capacitor charge causes an increasing electric field, which induces a circular magnetic field. Slide 34-50 Displacement Current d d(EA)d(q / ε) 1 dq E 0 dt dt dt ε0 dt dq d ε E dt0 dt The displacement current is equal to the conduction current!!! Bsd μ I μ ε I o o o d Maxwell’s Equations The First Unified Field Theory In his unified theory of electromagnetism, Maxwell showed that electromagnetic waves are a natural consequence of the fundamental laws expressed in these four equations: q EABAdd 0 εo dd Edd s BE B s μ I μ ε dto o o dt QuickCheck 34.4 The electric field is increasing. -

Fine Fuel Heating by Radiant Flux
Combust. Sci. and Tech., 182: 215–230, 2010 Copyright # Taylor & Francis Group, LLC ISSN: 0010-2202 print=1563-521X online DOI: 10.1080/00102200903341538 FINE FUEL HEATING BY RADIANT FLUX David Frankman,1 Brent W. Webb,1 Bret W. Butler,2 and Don J. Latham2 1Department of Mechanical Engineering, Brigham Young University, Provo, Utah, USA 2Rocky Mountain Research Station, U.S. Forest Service Fire Sciences Laboratory, Missoula, Montana, USA Experiments were conducted wherein wood shavings and Ponderosa pine needles in quiescent air were subjected to a steady radiation heat flux from a planar ceramic burner. The internal temperature of these particles was measured using fine diameter (0.076 mm diameter) type K thermocouples. A narrow angle radiometer was used to determine the emissive power generated by the burner. A model was developed to predict the steady-state temperature of a cylindrical particle with an imposed radiation heat flux under both quiesc- ent air (buoyancy-induced cooling) and windy (forced convection cooling) conditions. Excellent agreement was observed between the model predictions and the experimental data. Parametric studies using the validated model explore the effect of burner (flame) tem- perature and distance, fuel size, and wind speed. The data suggest that ignition of the fuel element by radiation heating alone is likely only under circumstances where the fire is very intense (such as crown fires), and even then may still be dependent on pilot ignition sources. Keywords: Fine fuel; Heating; Radiation INTRODUCTION Radiation and convection heat transfer have complimentary roles in wildland fire spread (Anderson, 1969), but due to the complexity of the wildland fire environ- ment, they remain largely undetermined. -

Radiant Energy Definition: the Energy of Electromagnetic Waves; This Includes Radio, Microwave, Infrared, Visible, Ultraviolet, X-Ray, and Gamma Waves
Radiant Energy Definition: the energy of electromagnetic waves; this includes radio, microwave, infrared, visible, ultraviolet, x-ray, and gamma waves Radiant Energy Solar panels Examples of energy transformations: Examples: • Photosynthesis: light from the • X-rays in medicine sun (RADIANT) converted to • Microwaves used to heat chemical energy in plants food • Heating food: electrical energy • Car radios and TV stations to mechanical and RADIANT • Cancer-causing UV rays energy to thermal energy and • Gamma rays from nuclear chemical energy. reactions Electrical Energy Definition: the energy of moving electrons. Electrical Energy Examples of energy Examples: transformations: • Electricity going through a • Making toast – ELECTRICAL to wire mechanical to radiant and • Lightning thermal to chemical. • Electric Discharge (shock) • Using a flashlight – chemical to ELECTRICAL to radiant and thermal. Chemical Energy: the energy required to make or break atom bonds; has the potential to go through a chemical change www.phschool.com Chemical Energy Energy Transformations using Chemical Energy: • Using a battery - the CHEMICAL energy is converted into Electrical energy Examples: • Eating Food – the CHEMICAL • batteries energy is converted into • Photosynthesis thermal energy and mechanical • Food energy Kinetic Energy Definition: the energy an object has because of its motion Kinetic Energy Examples of Kinetic Energy Forms: • Electrical • Mechanical • Thermal • Radiant Potential Energy Definition: the energy an object has because of its position or shape. Potential Energy Examples of Potential Energy Forms: • Gravitational Potential Energy • Chemical Energy • Elastic Energy • Nuclear Energy Mechanical Energy Definition: the total energy of motion and position of an object. Mechanical Energy= Potential Energy + Kinetic Energy Mechanical Energy Examples of energy Examples: transformations: • Coasting down a hill on • Exercising: Food (chemical) to your bike MECHANICAL and THERMAL as • Gears turning you move. -

A Review on Thermoelectric Generators: Progress and Applications
energies Review A Review on Thermoelectric Generators: Progress and Applications Mohamed Amine Zoui 1,2 , Saïd Bentouba 2 , John G. Stocholm 3 and Mahmoud Bourouis 4,* 1 Laboratory of Energy, Environment and Information Systems (LEESI), University of Adrar, Adrar 01000, Algeria; [email protected] 2 Laboratory of Sustainable Development and Computing (LDDI), University of Adrar, Adrar 01000, Algeria; [email protected] 3 Marvel Thermoelectrics, 11 rue Joachim du Bellay, 78540 Vernouillet, Île de France, France; [email protected] 4 Department of Mechanical Engineering, Universitat Rovira i Virgili, Av. Països Catalans No. 26, 43007 Tarragona, Spain * Correspondence: [email protected] Received: 7 June 2020; Accepted: 7 July 2020; Published: 13 July 2020 Abstract: A thermoelectric effect is a physical phenomenon consisting of the direct conversion of heat into electrical energy (Seebeck effect) or inversely from electrical current into heat (Peltier effect) without moving mechanical parts. The low efficiency of thermoelectric devices has limited their applications to certain areas, such as refrigeration, heat recovery, power generation and renewable energy. However, for specific applications like space probes, laboratory equipment and medical applications, where cost and efficiency are not as important as availability, reliability and predictability, thermoelectricity offers noteworthy potential. The challenge of making thermoelectricity a future leader in waste heat recovery and renewable energy is intensified by the integration of nanotechnology. In this review, state-of-the-art thermoelectric generators, applications and recent progress are reported. Fundamental knowledge of the thermoelectric effect, basic laws, and parameters affecting the efficiency of conventional and new thermoelectric materials are discussed. The applications of thermoelectricity are grouped into three main domains. -

The Human Ear Hearing, Sound Intensity and Loudness Levels
UIUC Physics 406 Acoustical Physics of Music The Human Ear Hearing, Sound Intensity and Loudness Levels We’ve been discussing the generation of sounds, so now we’ll discuss the perception of sounds. Human Senses: The astounding ~ 4 billion year evolution of living organisms on this planet, from the earliest single-cell life form(s) to the present day, with our current abilities to hear / see / smell / taste / feel / etc. – all are the result of the evolutionary forces of nature associated with “survival of the fittest” – i.e. it is evolutionarily{very} beneficial for us to be able to hear/perceive the natural sounds that do exist in the environment – it helps us to locate/find food/keep from becoming food, etc., just as vision/sight enables us to perceive objects in our 3-D environment, the ability to move /locomote through the environment enhances our ability to find food/keep from becoming food; Our sense of balance, via a stereo-pair (!) of semi-circular canals (= inertial guidance system!) helps us respond to 3-D inertial forces (e.g. gravity) and maintain our balance/avoid injury, etc. Our sense of taste & smell warn us of things that are bad to eat and/or breathe… Human Perception of Sound: * The human ear responds to disturbances/temporal variations in pressure. Amazingly sensitive! It has more than 6 orders of magnitude in dynamic range of pressure sensitivity (12 orders of magnitude in sound intensity, I p2) and 3 orders of magnitude in frequency (20 Hz – 20 KHz)! * Existence of 2 ears (stereo!) greatly enhances 3-D localization of sounds, and also the determination of pitch (i.e. -

Quantum and Thermal Conversion of Solar Energy to Useful Work
SERI/TP-252-2137 UC Category: 62e DE84000085 Quantum and Thermal Conversion of Solar Energy to Useful Work D. Johnson H. December 1983 Presented at the Solar Thermal Workshop Atlanta, Georgia 7-8 September 1983 Prepared under Task No. 1387.25 FTP No. 417 Solar Energy Research Institute ' A oivision of Midwest Research Institute 1617 Cole Bouievard Golden, Colorado 80401 Prepared for the U.S. Department of Energy Contract No. DE-AC02-83CH1 0093 NOTICE This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. TP-2137 QUANTUM AND THERMAL CONVERSION OF SOLAR ENERGY TO USEFUL WORK D. H. Johnson 1.0 INTRODUCTION This paper will summarize the results of a thermodynamic analysis of quantum and thermal processes for converting sunlight into useful work. Quantum and thermal processes acting alone as well as combined quantum-thermal processes will be discussed. Two types of combined processes have been analyzed, the thermally coupled process and the thermally decoupled process. These pro cesses were addressed because there is a hope that a combined quantum-thermal conversion system will prove to be cheaper than either system acting sepa rately. A first step in determining cost is to determine maximum system effi ciency. -

Thermal Energy
Thermal Energy By the Law of Conservation of Mechanical Energy, the work we put into a system is equal to the work we get out.If a ball is dropped from a height of 2 m, does it bounce back to its original height? No! The difference between the height dropped and the height of the bounce is the energy lost. Where did the energy go? Energy to compress the ball. Energy to overcome air resistance. And, the temperature of the ball increased (energy lost as heat) because of friction of the ball coming into contact with the floor.. Thermodynamics - The Study of Heat Heat is energy. More specific, heat is the flow of thermal energy between two objects. The unit of heat flow is the joule. If 1 g of water is raised 1 oC, 4.18 joules of heat is used. Or, if 1 kg (1 liter) of water is raised 1 oC, 4180 joules of heat energy is used. Another, more familiar unit for energy is the calorie. 4.18 joules = 1 calorie. For this course we will use 4180 j/kg oC for the specific heat capacity of water. Problem: How much heat energy (in joules) is used to raise the temperature of 25.0 g of water 10.0 oC? o Heat used (joules) = (mass H2O) x (specific heat of water) x (change in temp. C) # joules = (0.0250 kg) x (4180 j/kg oC) x (10.0 oC) = 1050 joules The English system uses British Thermal Units (BTU). The amount of energy needed to change the temperature of 1 pound of water by 1oF.