Chapter 2 Nature of Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the Linkage Between Planck's Quantum and Maxwell's Equations
The Finnish Society for Natural Philosophy 25 years, K.V. Laurikainen Honorary Symposium, Helsinki 11.-12.11.2013 On the Linkage between Planck's Quantum and Maxwell's Equations Tuomo Suntola Physics Foundations Society, www.physicsfoundations.org Abstract The concept of quantum is one of the key elements of the foundations in modern physics. The term quantum is primarily identified as a discrete amount of something. In the early 20th century, the concept of quantum was needed to explain Max Planck’s blackbody radiation law which suggested that the elec- tromagnetic radiation emitted by atomic oscillators at the surfaces of a blackbody cavity appears as dis- crete packets with the energy proportional to the frequency of the radiation in the packet. The derivation of Planck’s equation from Maxwell’s equations shows quantizing as a property of the emission/absorption process rather than an intrinsic property of radiation; the Planck constant becomes linked to primary electrical constants allowing a unified expression of the energies of mass objects and electromagnetic radiation thereby offering a novel insight into the wave nature of matter. From discrete atoms to a quantum of radiation Continuity or discontinuity of matter has been seen as a fundamental question since antiquity. Aristotle saw perfection in continuity and opposed Democritus’s ideas of indivisible atoms. First evidences of atoms and molecules were obtained in chemistry as multiple proportions of elements in chemical reac- tions by the end of the 18th and in the early 19th centuries. For physicist, the idea of atoms emerged for more than half a century later, most concretely in statistical thermodynamics and kinetic gas theory that converted the mole-based considerations of chemists into molecule-based considerations based on con- servation laws and probabilities. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -
Properties of Electromagnetic Waves Any Electromagnetic Wave Must Satisfy Four Basic Conditions: 1
Chapter 34 Electromagnetic Waves The Goal of the Entire Course Maxwell’s Equations: Maxwell’s Equations James Clerk Maxwell •1831 – 1879 •Scottish theoretical physicist •Developed the electromagnetic theory of light •His successful interpretation of the electromagnetic field resulted in the field equations that bear his name. •Also developed and explained – Kinetic theory of gases – Nature of Saturn’s rings – Color vision Start at 12:50 https://www.learner.org/vod/vod_window.html?pid=604 Correcting Ampere’s Law Two surfaces S1 and S2 near the plate of a capacitor are bounded by the same path P. Ampere’s Law states that But it is zero on S2 since there is no conduction current through it. This is a contradiction. Maxwell fixed it by introducing the displacement current: Fig. 34-1, p. 984 Maxwell hypothesized that a changing electric field creates an induced magnetic field. Induced Fields . An increasing solenoid current causes an increasing magnetic field, which induces a circular electric field. An increasing capacitor charge causes an increasing electric field, which induces a circular magnetic field. Slide 34-50 Displacement Current d d(EA)d(q / ε) 1 dq E 0 dt dt dt ε0 dt dq d ε E dt0 dt The displacement current is equal to the conduction current!!! Bsd μ I μ ε I o o o d Maxwell’s Equations The First Unified Field Theory In his unified theory of electromagnetism, Maxwell showed that electromagnetic waves are a natural consequence of the fundamental laws expressed in these four equations: q EABAdd 0 εo dd Edd s BE B s μ I μ ε dto o o dt QuickCheck 34.4 The electric field is increasing. -

Turbulence Examined in the Frequency-Wavenumber Domain*
Turbulence examined in the frequency-wavenumber domain∗ Andrew J. Morten Department of Physics University of Michigan Supported by funding from: National Science Foundation and Office of Naval Research ∗ Arbic et al. JPO 2012; Arbic et al. in review; Morten et al. papers in preparation Andrew J. Morten LOM 2013, Ann Arbor, MI Collaborators • University of Michigan Brian Arbic, Charlie Doering • MIT Glenn Flierl • University of Brest, and The University of Texas at Austin Robert Scott Andrew J. Morten LOM 2013, Ann Arbor, MI Outline of talk Part I{Motivation (research led by Brian Arbic) • Frequency-wavenumber analysis: {Idealized Quasi-geostrophic (QG) turbulence model. {High-resolution ocean general circulation model (HYCOM)∗: {AVISO gridded satellite altimeter data. ∗We used NLOM in Arbic et al. (2012) Part II-Research led by Andrew Morten • Derivation and interpretation of spectral transfers used above. • Frequency-domain analysis in two-dimensional turbulence. • Theoretical prediction for frequency spectra and spectral transfers due to the effects of \sweeping." {moving beyond a zeroth order approximation. Andrew J. Morten LOM 2013, Ann Arbor, MI Motivation: Intrinsic oceanic variability • Interested in quantifying the contributions of intrinsic nonlinearities in oceanic dynamics to oceanic frequency spectra. • Penduff et al. 2011: Interannual SSH variance in ocean models with interannual atmospheric forcing is comparable to variance in high resolution (eddying) ocean models with no interannual atmospheric forcing. • Might this eddy-driven low-frequency variability be connected to the well-known inverse cascade to low wavenumbers (e.g. Fjortoft 1953)? • A separate motivation is simply that transfers in mixed ! − k space provide a useful diagnostic. Andrew J. -

Variable Planck's Constant
Variable Planck’s Constant: Treated As A Dynamical Field And Path Integral Rand Dannenberg Ventura College, Physics and Astronomy Department, Ventura CA [email protected] [email protected] January 28, 2021 Abstract. The constant ħ is elevated to a dynamical field, coupling to other fields, and itself, through the Lagrangian density derivative terms. The spatial and temporal dependence of ħ falls directly out of the field equations themselves. Three solutions are found: a free field with a tadpole term; a standing-wave non-propagating mode; a non-oscillating non-propagating mode. The first two could be quantized. The third corresponds to a zero-momentum classical field that naturally decays spatially to a constant with no ad-hoc terms added to the Lagrangian. An attempt is made to calibrate the constants in the third solution based on experimental data. The three fields are referred to as actons. It is tentatively concluded that the acton origin coincides with a massive body, or point of infinite density, though is not mass dependent. An expression for the positional dependence of Planck’s constant is derived from a field theory in this work that matches in functional form that of one derived from considerations of Local Position Invariance violation in GR in another paper by this author. Astrophysical and Cosmological interpretations are provided. A derivation is shown for how the integrand in the path integral exponent becomes Lc/ħ(r), where Lc is the classical action. The path that makes stationary the integral in the exponent is termed the “dominant” path, and deviates from the classical path systematically due to the position dependence of ħ. -

Variable Planck's Constant
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 29 January 2021 doi:10.20944/preprints202101.0612.v1 Variable Planck’s Constant: Treated As A Dynamical Field And Path Integral Rand Dannenberg Ventura College, Physics and Astronomy Department, Ventura CA [email protected] Abstract. The constant ħ is elevated to a dynamical field, coupling to other fields, and itself, through the Lagrangian density derivative terms. The spatial and temporal dependence of ħ falls directly out of the field equations themselves. Three solutions are found: a free field with a tadpole term; a standing-wave non-propagating mode; a non-oscillating non-propagating mode. The first two could be quantized. The third corresponds to a zero-momentum classical field that naturally decays spatially to a constant with no ad-hoc terms added to the Lagrangian. An attempt is made to calibrate the constants in the third solution based on experimental data. The three fields are referred to as actons. It is tentatively concluded that the acton origin coincides with a massive body, or point of infinite density, though is not mass dependent. An expression for the positional dependence of Planck’s constant is derived from a field theory in this work that matches in functional form that of one derived from considerations of Local Position Invariance violation in GR in another paper by this author. Astrophysical and Cosmological interpretations are provided. A derivation is shown for how the integrand in the path integral exponent becomes Lc/ħ(r), where Lc is the classical action. The path that makes stationary the integral in the exponent is termed the “dominant” path, and deviates from the classical path systematically due to the position dependence of ħ. -

The Principle of Relativity and the De Broglie Relation Abstract
The principle of relativity and the De Broglie relation Julio G¨u´emez∗ Department of Applied Physics, Faculty of Sciences, University of Cantabria, Av. de Los Castros, 39005 Santander, Spain Manuel Fiolhaisy Physics Department and CFisUC, University of Coimbra, 3004-516 Coimbra, Portugal Luis A. Fern´andezz Department of Mathematics, Statistics and Computation, Faculty of Sciences, University of Cantabria, Av. de Los Castros, 39005 Santander, Spain (Dated: February 5, 2016) Abstract The De Broglie relation is revisited in connection with an ab initio relativistic description of particles and waves, which was the same treatment that historically led to that famous relation. In the same context of the Minkowski four-vectors formalism, we also discuss the phase and the group velocity of a matter wave, explicitly showing that, under a Lorentz transformation, both transform as ordinary velocities. We show that such a transformation rule is a necessary condition for the covariance of the De Broglie relation, and stress the pedagogical value of the Einstein-Minkowski- Lorentz relativistic context in the presentation of the De Broglie relation. 1 I. INTRODUCTION The motivation for this paper is to emphasize the advantage of discussing the De Broglie relation in the framework of special relativity, formulated with Minkowski's four-vectors, as actually was done by De Broglie himself.1 The De Broglie relation, usually written as h λ = ; (1) p which introduces the concept of a wavelength λ, for a \material wave" associated with a massive particle (such as an electron), with linear momentum p and h being Planck's constant. Equation (1) is typically presented in the first or the second semester of a physics major, in a curricular unit of introduction to modern physics. -
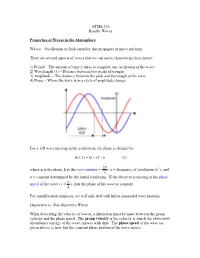
ATMS 310 Rossby Waves Properties of Waves in the Atmosphere Waves
ATMS 310 Rossby Waves Properties of Waves in the Atmosphere Waves – Oscillations in field variables that propagate in space and time. There are several aspects of waves that we can use to characterize their nature: 1) Period – The amount of time it takes to complete one oscillation of the wave\ 2) Wavelength (λ) – Distance between two peaks of troughs 3) Amplitude – The distance between the peak and the trough of the wave 4) Phase – Where the wave is in a cycle of amplitude change For a 1-D wave moving in the x-direction, the phase is defined by: φ ),( = −υtkxtx − α (1) 2π where φ is the phase, k is the wave number = , υ = frequency of oscillation (s-1), and λ α = constant determined by the initial conditions. If the observer is moving at the phase υ speed of the wave ( c ≡ ), then the phase of the wave is constant. k For simplification purposes, we will only deal with linear sinusoidal wave motions. Dispersive vs. Non-dispersive Waves When describing the velocity of waves, a distinction must be made between the group velocity and the phase speed. The group velocity is the velocity at which the observable disturbance (energy of the wave) moves with time. The phase speed of the wave (as given above) is how fast the constant phase portion of the wave moves. A dispersive wave is one in which the pattern of the wave changes with time. In dispersive waves, the group velocity is usually different than the phase speed. A non- dispersive wave is one in which the patterns of the wave do not change with time as the wave propagates (“rigid” wave). -

The Human Ear Hearing, Sound Intensity and Loudness Levels
UIUC Physics 406 Acoustical Physics of Music The Human Ear Hearing, Sound Intensity and Loudness Levels We’ve been discussing the generation of sounds, so now we’ll discuss the perception of sounds. Human Senses: The astounding ~ 4 billion year evolution of living organisms on this planet, from the earliest single-cell life form(s) to the present day, with our current abilities to hear / see / smell / taste / feel / etc. – all are the result of the evolutionary forces of nature associated with “survival of the fittest” – i.e. it is evolutionarily{very} beneficial for us to be able to hear/perceive the natural sounds that do exist in the environment – it helps us to locate/find food/keep from becoming food, etc., just as vision/sight enables us to perceive objects in our 3-D environment, the ability to move /locomote through the environment enhances our ability to find food/keep from becoming food; Our sense of balance, via a stereo-pair (!) of semi-circular canals (= inertial guidance system!) helps us respond to 3-D inertial forces (e.g. gravity) and maintain our balance/avoid injury, etc. Our sense of taste & smell warn us of things that are bad to eat and/or breathe… Human Perception of Sound: * The human ear responds to disturbances/temporal variations in pressure. Amazingly sensitive! It has more than 6 orders of magnitude in dynamic range of pressure sensitivity (12 orders of magnitude in sound intensity, I p2) and 3 orders of magnitude in frequency (20 Hz – 20 KHz)! * Existence of 2 ears (stereo!) greatly enhances 3-D localization of sounds, and also the determination of pitch (i.e. -
Molecular Spectroscopy
Solutions manual for Burrows et.al. Chemistry3 Third edition 10 Molecular spectroscopy Answers to worked examples W.E. 10.1 Using the Beer-Lambert law (on p. 462 in Chemistry3) What concentration of the solution is required to absorb 35% of the light at the same wavelength in an identical cell? Strategy Rearrange Equation 10.8 to give an expression for the concentration of the solution in terms of the absorbance, path length and absorption coefficient. Calculate the absorbance and substitute, along with the values given in the example. Solution The absorbance of the solution is given by Equation 10.7 퐴 = log10(퐼0/퐼t) = log10(1.00/0.65) = +0.187 because if 35% of the incident radiation is absorbed, 65% must be transmitted. We may rearrange Equation 10.8 and substitute the values directly 푐 = 퐴/(휖푙) = +0.187/{(15.1 m2mol−1) × (0.0100 m)} = 1.24 mol m−3 = 1.24 × 10−3mol dm−3 W.E. 10.2 Using the Boltzmann distribution (on p. 463 in Chemistry3) Calculate the relative populations of the two energy levels, each with a degeneracy of one, separated by 5.0 kJ mol1 at: (a) 200 K; (b) 300 K; (c) 500 K. What is the effect of temperature on the relative populations in the two levels? H i g h e r E d u c a t i o n © Oxford University Press, 2017. All rights reserved. Solutions manual for Burrows et.al. Chemistry3 Third edition Strategy Use Equation 10.9, which shows how the Boltzmann distribution affects the relative populations of two energy levels. -

Reflection and Refraction of Light
Reflection and refraction When light hits a surface, one or more of three things may happen. The light may be reflected back from the surface, transmitted through the material (in which case it will deviate from its initial direction, a process known as refraction) or absorbed by the material if it is not transparent at the wavelength of the incident light. 1. Reflection and refraction Reflection and refraction are governed by two very simple laws. We consider a light wave travelling through medium 1 and striking medium 2. We define the angle of incidence θ as the angle between the incident ray and the normal to the surface (a vector pointing out perpendicular to the surface). For reflection, the reflected ray lies in the plane of incidence, and θ1’ = θ1 For refraction, the refracted ray lies in the plane of incidence, and n1sinθ1 = n2sinθ2 (this expression is called Snell’s law). In the above equations, ni is a dimensionless constant known as the θ1 θ1’ index of refraction or refractive index of medium i. In addition to reflection determining the angle of refraction, n also determines the speed of the light wave in the medium, v = c/n. Just as θ1 is the angle between refraction θ2 the incident ray and the surface normal outside the medium, θ2 is the angle between the transmitted ray and the surface normal inside the medium (i.e. pointing out from the other side of the surface to the original surface normal) 2. Total internal reflection A consequence of Snell’s law is a phenomenon known as total internal reflection.