Λ = H/P (De Broglie’S Hypothesis) Where P Is the Momentum and H Is the Same Planck’S Constant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

Non-Einsteinian Interpretations of the Photoelectric Effect
NON-EINSTEINIAN INTERPRETATIONS ------ROGER H. STUEWER------ serveq spectral distribution for black-body radiation, and it led inexorably ~o _the ~latantl~ f::se conclusion that the total radiant energy in a cavity is mfimte. (This ultra-violet catastrophe," as Ehrenfest later termed it, was point~d out independently and virtually simultaneously, though not Non-Einsteinian Interpretations of the as emphatically, by Lord Rayleigh.2) Photoelectric Effect Einstein developed his positive argument in two stages. First, he derived an expression for the entropy of black-body radiation in the Wien's law spectral region (the high-frequency, low-temperature region) and used this expression to evaluate the difference in entropy associated with a chan.ge in volume v - Vo of the radiation, at constant energy. Secondly, he Few, if any, ideas in the history of recent physics were greeted with ~o~s1dere? the case of n particles freely and independently moving around skepticism as universal and prolonged as was Einstein's light quantum ms1d: a given v~lume Vo _and determined the change in entropy of the sys hypothesis.1 The period of transition between rejection and acceptance tem if all n particles accidentally found themselves in a given subvolume spanned almost two decades and would undoubtedly have been longer if v ~ta r~ndomly chosen instant of time. To evaluate this change in entropy, Arthur Compton's experiments had been less striking. Why was Einstein's Emstem used the statistical version of the second law of thermodynamics hypothesis not accepted much earlier? Was there no experimental evi in _c?njunction with his own strongly physical interpretation of the prob dence that suggested, even demanded, Einstein's hypothesis? If so, why ~b1ht~. -

On the Linkage Between Planck's Quantum and Maxwell's Equations
The Finnish Society for Natural Philosophy 25 years, K.V. Laurikainen Honorary Symposium, Helsinki 11.-12.11.2013 On the Linkage between Planck's Quantum and Maxwell's Equations Tuomo Suntola Physics Foundations Society, www.physicsfoundations.org Abstract The concept of quantum is one of the key elements of the foundations in modern physics. The term quantum is primarily identified as a discrete amount of something. In the early 20th century, the concept of quantum was needed to explain Max Planck’s blackbody radiation law which suggested that the elec- tromagnetic radiation emitted by atomic oscillators at the surfaces of a blackbody cavity appears as dis- crete packets with the energy proportional to the frequency of the radiation in the packet. The derivation of Planck’s equation from Maxwell’s equations shows quantizing as a property of the emission/absorption process rather than an intrinsic property of radiation; the Planck constant becomes linked to primary electrical constants allowing a unified expression of the energies of mass objects and electromagnetic radiation thereby offering a novel insight into the wave nature of matter. From discrete atoms to a quantum of radiation Continuity or discontinuity of matter has been seen as a fundamental question since antiquity. Aristotle saw perfection in continuity and opposed Democritus’s ideas of indivisible atoms. First evidences of atoms and molecules were obtained in chemistry as multiple proportions of elements in chemical reac- tions by the end of the 18th and in the early 19th centuries. For physicist, the idea of atoms emerged for more than half a century later, most concretely in statistical thermodynamics and kinetic gas theory that converted the mole-based considerations of chemists into molecule-based considerations based on con- servation laws and probabilities. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Turbulence Examined in the Frequency-Wavenumber Domain*
Turbulence examined in the frequency-wavenumber domain∗ Andrew J. Morten Department of Physics University of Michigan Supported by funding from: National Science Foundation and Office of Naval Research ∗ Arbic et al. JPO 2012; Arbic et al. in review; Morten et al. papers in preparation Andrew J. Morten LOM 2013, Ann Arbor, MI Collaborators • University of Michigan Brian Arbic, Charlie Doering • MIT Glenn Flierl • University of Brest, and The University of Texas at Austin Robert Scott Andrew J. Morten LOM 2013, Ann Arbor, MI Outline of talk Part I{Motivation (research led by Brian Arbic) • Frequency-wavenumber analysis: {Idealized Quasi-geostrophic (QG) turbulence model. {High-resolution ocean general circulation model (HYCOM)∗: {AVISO gridded satellite altimeter data. ∗We used NLOM in Arbic et al. (2012) Part II-Research led by Andrew Morten • Derivation and interpretation of spectral transfers used above. • Frequency-domain analysis in two-dimensional turbulence. • Theoretical prediction for frequency spectra and spectral transfers due to the effects of \sweeping." {moving beyond a zeroth order approximation. Andrew J. Morten LOM 2013, Ann Arbor, MI Motivation: Intrinsic oceanic variability • Interested in quantifying the contributions of intrinsic nonlinearities in oceanic dynamics to oceanic frequency spectra. • Penduff et al. 2011: Interannual SSH variance in ocean models with interannual atmospheric forcing is comparable to variance in high resolution (eddying) ocean models with no interannual atmospheric forcing. • Might this eddy-driven low-frequency variability be connected to the well-known inverse cascade to low wavenumbers (e.g. Fjortoft 1953)? • A separate motivation is simply that transfers in mixed ! − k space provide a useful diagnostic. Andrew J. -

Variable Planck's Constant
Variable Planck’s Constant: Treated As A Dynamical Field And Path Integral Rand Dannenberg Ventura College, Physics and Astronomy Department, Ventura CA [email protected] [email protected] January 28, 2021 Abstract. The constant ħ is elevated to a dynamical field, coupling to other fields, and itself, through the Lagrangian density derivative terms. The spatial and temporal dependence of ħ falls directly out of the field equations themselves. Three solutions are found: a free field with a tadpole term; a standing-wave non-propagating mode; a non-oscillating non-propagating mode. The first two could be quantized. The third corresponds to a zero-momentum classical field that naturally decays spatially to a constant with no ad-hoc terms added to the Lagrangian. An attempt is made to calibrate the constants in the third solution based on experimental data. The three fields are referred to as actons. It is tentatively concluded that the acton origin coincides with a massive body, or point of infinite density, though is not mass dependent. An expression for the positional dependence of Planck’s constant is derived from a field theory in this work that matches in functional form that of one derived from considerations of Local Position Invariance violation in GR in another paper by this author. Astrophysical and Cosmological interpretations are provided. A derivation is shown for how the integrand in the path integral exponent becomes Lc/ħ(r), where Lc is the classical action. The path that makes stationary the integral in the exponent is termed the “dominant” path, and deviates from the classical path systematically due to the position dependence of ħ. -

1. Physical Constants 1 1
1. Physical constants 1 1. PHYSICAL CONSTANTS Table 1.1. Reviewed 1998 by B.N. Taylor (NIST). Based mainly on the “1986 Adjustment of the Fundamental Physical Constants” by E.R. Cohen and B.N. Taylor, Rev. Mod. Phys. 59, 1121 (1987). The last group of constants (beginning with the Fermi coupling constant) comes from the Particle Data Group. The figures in parentheses after the values give the 1-standard- deviation uncertainties in the last digits; the corresponding uncertainties in parts per million (ppm) are given in the last column. This set of constants (aside from the last group) is recommended for international use by CODATA (the Committee on Data for Science and Technology). Since the 1986 adjustment, new experiments have yielded improved values for a number of constants, including the Rydberg constant R∞, the Planck constant h, the fine- structure constant α, and the molar gas constant R,and hence also for constants directly derived from these, such as the Boltzmann constant k and Stefan-Boltzmann constant σ. The new results and their impact on the 1986 recommended values are discussed extensively in “Recommended Values of the Fundamental Physical Constants: A Status Report,” B.N. Taylor and E.R. Cohen, J. Res. Natl. Inst. Stand. Technol. 95, 497 (1990); see also E.R. Cohen and B.N. Taylor, “The Fundamental Physical Constants,” Phys. Today, August 1997 Part 2, BG7. In general, the new results give uncertainties for the affected constants that are 5 to 7 times smaller than the 1986 uncertainties, but the changes in the values themselves are smaller than twice the 1986 uncertainties. -

Variable Planck's Constant
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 29 January 2021 doi:10.20944/preprints202101.0612.v1 Variable Planck’s Constant: Treated As A Dynamical Field And Path Integral Rand Dannenberg Ventura College, Physics and Astronomy Department, Ventura CA [email protected] Abstract. The constant ħ is elevated to a dynamical field, coupling to other fields, and itself, through the Lagrangian density derivative terms. The spatial and temporal dependence of ħ falls directly out of the field equations themselves. Three solutions are found: a free field with a tadpole term; a standing-wave non-propagating mode; a non-oscillating non-propagating mode. The first two could be quantized. The third corresponds to a zero-momentum classical field that naturally decays spatially to a constant with no ad-hoc terms added to the Lagrangian. An attempt is made to calibrate the constants in the third solution based on experimental data. The three fields are referred to as actons. It is tentatively concluded that the acton origin coincides with a massive body, or point of infinite density, though is not mass dependent. An expression for the positional dependence of Planck’s constant is derived from a field theory in this work that matches in functional form that of one derived from considerations of Local Position Invariance violation in GR in another paper by this author. Astrophysical and Cosmological interpretations are provided. A derivation is shown for how the integrand in the path integral exponent becomes Lc/ħ(r), where Lc is the classical action. The path that makes stationary the integral in the exponent is termed the “dominant” path, and deviates from the classical path systematically due to the position dependence of ħ. -

Determination of Planck's Constant Using the Photoelectric Effect
Determination of Planck’s Constant Using the Photoelectric Effect Lulu Liu (Partner: Pablo Solis)∗ MIT Undergraduate (Dated: September 25, 2007) Together with my partner, Pablo Solis, we demonstrate the particle-like nature of light as charac- terized by photons with quantized and distinct energies each dependent only on its frequency (ν) and scaled by Planck’s constant (h). Using data obtained through the bombardment of an alkali metal surface (in our case, potassium) by light of varying frequencies, we calculate Planck’s constant, h to be 1.92 × 10−15 ± 1.08 × 10−15eV · s. 1. THEORY AND MOTIVATION This maximum kinetic energy can be determined by applying a retarding potential (Vr) across a vacuum gap It was discovered by Heinrich Hertz that light incident in a circuit with an amp meter. On one side of this gap upon a matter target caused the emission of electrons is the photoelectron emitter, a metal with work function from the target. The effect was termed the Hertz Effect W0. We let light of different frequencies strike this emit- (and later the Photoelectric Effect) and the electrons re- ter. When eVr = Kmax we will cease to see any current ferred to as photoelectrons. It was understood that the through the circuit. By finding this voltage we calculate electrons were able to absorb the energy of the incident the maximum kinetic energy of the electrons emitted as a light and escape from the coulomb potential that bound function of radiation frequency. This relationship (with it to the nucleus. some variation due to error) should model Equation 2 According to classical wave theory, the energy of a above. -

The Principle of Relativity and the De Broglie Relation Abstract
The principle of relativity and the De Broglie relation Julio G¨u´emez∗ Department of Applied Physics, Faculty of Sciences, University of Cantabria, Av. de Los Castros, 39005 Santander, Spain Manuel Fiolhaisy Physics Department and CFisUC, University of Coimbra, 3004-516 Coimbra, Portugal Luis A. Fern´andezz Department of Mathematics, Statistics and Computation, Faculty of Sciences, University of Cantabria, Av. de Los Castros, 39005 Santander, Spain (Dated: February 5, 2016) Abstract The De Broglie relation is revisited in connection with an ab initio relativistic description of particles and waves, which was the same treatment that historically led to that famous relation. In the same context of the Minkowski four-vectors formalism, we also discuss the phase and the group velocity of a matter wave, explicitly showing that, under a Lorentz transformation, both transform as ordinary velocities. We show that such a transformation rule is a necessary condition for the covariance of the De Broglie relation, and stress the pedagogical value of the Einstein-Minkowski- Lorentz relativistic context in the presentation of the De Broglie relation. 1 I. INTRODUCTION The motivation for this paper is to emphasize the advantage of discussing the De Broglie relation in the framework of special relativity, formulated with Minkowski's four-vectors, as actually was done by De Broglie himself.1 The De Broglie relation, usually written as h λ = ; (1) p which introduces the concept of a wavelength λ, for a \material wave" associated with a massive particle (such as an electron), with linear momentum p and h being Planck's constant. Equation (1) is typically presented in the first or the second semester of a physics major, in a curricular unit of introduction to modern physics. -
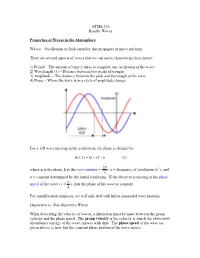
ATMS 310 Rossby Waves Properties of Waves in the Atmosphere Waves
ATMS 310 Rossby Waves Properties of Waves in the Atmosphere Waves – Oscillations in field variables that propagate in space and time. There are several aspects of waves that we can use to characterize their nature: 1) Period – The amount of time it takes to complete one oscillation of the wave\ 2) Wavelength (λ) – Distance between two peaks of troughs 3) Amplitude – The distance between the peak and the trough of the wave 4) Phase – Where the wave is in a cycle of amplitude change For a 1-D wave moving in the x-direction, the phase is defined by: φ ),( = −υtkxtx − α (1) 2π where φ is the phase, k is the wave number = , υ = frequency of oscillation (s-1), and λ α = constant determined by the initial conditions. If the observer is moving at the phase υ speed of the wave ( c ≡ ), then the phase of the wave is constant. k For simplification purposes, we will only deal with linear sinusoidal wave motions. Dispersive vs. Non-dispersive Waves When describing the velocity of waves, a distinction must be made between the group velocity and the phase speed. The group velocity is the velocity at which the observable disturbance (energy of the wave) moves with time. The phase speed of the wave (as given above) is how fast the constant phase portion of the wave moves. A dispersive wave is one in which the pattern of the wave changes with time. In dispersive waves, the group velocity is usually different than the phase speed. A non- dispersive wave is one in which the patterns of the wave do not change with time as the wave propagates (“rigid” wave).