Determination of Planck's Constant Using the Photoelectric Effect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

Non-Einsteinian Interpretations of the Photoelectric Effect
NON-EINSTEINIAN INTERPRETATIONS ------ROGER H. STUEWER------ serveq spectral distribution for black-body radiation, and it led inexorably ~o _the ~latantl~ f::se conclusion that the total radiant energy in a cavity is mfimte. (This ultra-violet catastrophe," as Ehrenfest later termed it, was point~d out independently and virtually simultaneously, though not Non-Einsteinian Interpretations of the as emphatically, by Lord Rayleigh.2) Photoelectric Effect Einstein developed his positive argument in two stages. First, he derived an expression for the entropy of black-body radiation in the Wien's law spectral region (the high-frequency, low-temperature region) and used this expression to evaluate the difference in entropy associated with a chan.ge in volume v - Vo of the radiation, at constant energy. Secondly, he Few, if any, ideas in the history of recent physics were greeted with ~o~s1dere? the case of n particles freely and independently moving around skepticism as universal and prolonged as was Einstein's light quantum ms1d: a given v~lume Vo _and determined the change in entropy of the sys hypothesis.1 The period of transition between rejection and acceptance tem if all n particles accidentally found themselves in a given subvolume spanned almost two decades and would undoubtedly have been longer if v ~ta r~ndomly chosen instant of time. To evaluate this change in entropy, Arthur Compton's experiments had been less striking. Why was Einstein's Emstem used the statistical version of the second law of thermodynamics hypothesis not accepted much earlier? Was there no experimental evi in _c?njunction with his own strongly physical interpretation of the prob dence that suggested, even demanded, Einstein's hypothesis? If so, why ~b1ht~. -

1. Physical Constants 1 1
1. Physical constants 1 1. PHYSICAL CONSTANTS Table 1.1. Reviewed 1998 by B.N. Taylor (NIST). Based mainly on the “1986 Adjustment of the Fundamental Physical Constants” by E.R. Cohen and B.N. Taylor, Rev. Mod. Phys. 59, 1121 (1987). The last group of constants (beginning with the Fermi coupling constant) comes from the Particle Data Group. The figures in parentheses after the values give the 1-standard- deviation uncertainties in the last digits; the corresponding uncertainties in parts per million (ppm) are given in the last column. This set of constants (aside from the last group) is recommended for international use by CODATA (the Committee on Data for Science and Technology). Since the 1986 adjustment, new experiments have yielded improved values for a number of constants, including the Rydberg constant R∞, the Planck constant h, the fine- structure constant α, and the molar gas constant R,and hence also for constants directly derived from these, such as the Boltzmann constant k and Stefan-Boltzmann constant σ. The new results and their impact on the 1986 recommended values are discussed extensively in “Recommended Values of the Fundamental Physical Constants: A Status Report,” B.N. Taylor and E.R. Cohen, J. Res. Natl. Inst. Stand. Technol. 95, 497 (1990); see also E.R. Cohen and B.N. Taylor, “The Fundamental Physical Constants,” Phys. Today, August 1997 Part 2, BG7. In general, the new results give uncertainties for the affected constants that are 5 to 7 times smaller than the 1986 uncertainties, but the changes in the values themselves are smaller than twice the 1986 uncertainties. -

Quantifying the Quantum Stephan Schlamminger Looks at the Origins of the Planck Constant and Its Current Role in Redefining the Kilogram
measure for measure Quantifying the quantum Stephan Schlamminger looks at the origins of the Planck constant and its current role in redefining the kilogram. child on a swing is an everyday mechanical constant (h) with high precision, measure a approximation of a macroscopic a macroscopic experiment is necessary, force — in A harmonic oscillator. The total energy because the unit of mass, the kilogram, can this case of such a system depends on its amplitude, only be accessed with high precision at the the weight while the frequency of oscillation is, at one-kilogram value through its definition of a mass least for small amplitudes, independent as the mass of the International Prototype m — as of the amplitude. So, it seems that the of the Kilogram (IPK). The IPK is the only the product energy of the system can be adjusted object on Earth whose mass we know for of a current continuously from zero upwards — that is, certain2 — all relative uncertainties increase and a voltage the child can swing at any amplitude. This from thereon. divided by a velocity, is not the case, however, for a microscopic The revised SI, likely to come into mg = UI/v (with g the oscillator, the physics of which is described effect in 2019, is based on fixed numerical Earth’s gravitational by the laws of quantum mechanics. A values, without uncertainties, of seven acceleration). quantum-mechanical swing can change defining constants, three of which are Meanwhile, the its energy only in steps of hν, the product already fixed in the present SI; the four discoveries of the Josephson of the Planck constant and the oscillation additional ones are the elementary and quantum Hall effects frequency — the (energy) quantum of charge and the Planck, led to precise electrical the oscillator. -

1. Physical Constants 101
1. Physical constants 101 1. PHYSICAL CONSTANTS Table 1.1. Reviewed 2010 by P.J. Mohr (NIST). Mainly from the “CODATA Recommended Values of the Fundamental Physical Constants: 2006” by P.J. Mohr, B.N. Taylor, and D.B. Newell in Rev. Mod. Phys. 80 (2008) 633. The last group of constants (beginning with the Fermi coupling constant) comes from the Particle Data Group. The figures in parentheses after the values give the 1-standard-deviation uncertainties in the last digits; the corresponding fractional uncertainties in parts per 109 (ppb) are given in the last column. This set of constants (aside from the last group) is recommended for international use by CODATA (the Committee on Data for Science and Technology). The full 2006 CODATA set of constants may be found at http://physics.nist.gov/constants. See also P.J. Mohr and D.B. Newell, “Resource Letter FC-1: The Physics of Fundamental Constants,” Am. J. Phys, 78 (2010) 338. Quantity Symbol, equation Value Uncertainty (ppb) speed of light in vacuum c 299 792 458 m s−1 exact∗ Planck constant h 6.626 068 96(33)×10−34 Js 50 Planck constant, reduced ≡ h/2π 1.054 571 628(53)×10−34 Js 50 = 6.582 118 99(16)×10−22 MeV s 25 electron charge magnitude e 1.602 176 487(40)×10−19 C = 4.803 204 27(12)×10−10 esu 25, 25 conversion constant c 197.326 9631(49) MeV fm 25 conversion constant (c)2 0.389 379 304(19) GeV2 mbarn 50 2 −31 electron mass me 0.510 998 910(13) MeV/c = 9.109 382 15(45)×10 kg 25, 50 2 −27 proton mass mp 938.272 013(23) MeV/c = 1.672 621 637(83)×10 kg 25, 50 = 1.007 276 466 77(10) u = 1836.152 672 47(80) me 0.10, 0.43 2 deuteron mass md 1875.612 793(47) MeV/c 25 12 2 −27 unified atomic mass unit (u) (mass C atom)/12 = (1 g)/(NA mol) 931.494 028(23) MeV/c = 1.660 538 782(83)×10 kg 25, 50 2 −12 −1 permittivity of free space 0 =1/μ0c 8.854 187 817 .. -

Λ = H/P (De Broglie’S Hypothesis) Where P Is the Momentum and H Is the Same Planck’S Constant
PHYSICS 2800 – 2nd TERM Outline Notes Section 1. Basics of Quantum Physics The approach here will be somewhat historical, emphasizing the main developments that are useful for the applications coming later for materials science. We begin with a brief (but incomplete history), just to put things into context. 1.1 Some historical background (exploring “wave” versus “particle” ideas) Before ~ 1800: Matter behaves as if continuous (seems to be infinitely divisible) Light is particle-like (shadows have sharp edges; lenses described using ray tracing). From ~ 1800 to ~ early1900s: Matter consists of lots of particles - measurement of e/m ratio for electron (JJ Thomson 1897) - measurement of e for electron (Millikan 1909; he deduced mass me was much smaller than matom ) - discovery of the nucleus (Rutherford 1913; he found that α particles could scatter at large angles from metal foil). Light is a wave - coherent light from two sources can interfere (Young 1801). Just after ~ 1900: Light is a particle (maybe)? - photoelectric effect (explained by Einstein 1905 assuming light consists of particles) - Compton effect (Compton 1923; he explained scattering of X-rays from electrons in terms of collisions with particles of light; needed relativity). Matter is a wave (maybe)? - matter has wavelike properties and can exhibit interference effects (de Broglie wave hypothesis 1923) - the Bohr atom (Bohr 1923: he developed a model for the H-atom with only certain stable states allowed → ideas of “quantization”) - interference effects shown for electrons and neutrons (now the basis of major experimental techniques). The present time: Both matter and light can have both particle and wavelike properties. -

The Planck Constant and the Origin of Mass Due to a Higher Order Casimir Effect
The Planck Constant and the Origin of Mass due to a Higher Order Casimir Effect C. Baumgartel¨ and M. Tajmar∗ Institute of Aerospace Engineering, Technische Universit¨atDresden, Germany (Dated: May 31, 2018) The Planck constant is one of the most important constants in nature, as it describes the world governed by quantum mechanics. However, it cannot be derived from other natural constants. We present a model from which it is possible to derive this constant without any free parameters. This is done utilizing the force between two oscillating electric dipoles described by an extension of Weber electrodynamics, based on a gravitational model by Assis. This leads not only to gravitational forces between the particles but also to a newly found Casimir-type attraction. We can use these forces to calculate the maximum point mass of this model which is equal to the Planck mass and derive the quantum of action. The result hints to a connection of quantum effects like the Casimir force and the Planck constant with gravitational ones and the origin of mass itself. Keywords: Planck Constant, Casimir Force, Quantum Gravity, Unified Field Theory, Weber Electrodynamics, Origin of Mass I. INTRODUCTION be predicted more accurately with Weber-type formulae than Maxwell-Lorentz ones. The quantum of action h is one of the fundamental constants This interest leads us to study further on this subject and in- of nature. Originally assumed to calculate the black body ra- vestigate the bonds that connect Weber’s law and nature’s phe- diation, its value has been determined experimentally to a high nomena, as this may be a possibility to find a long sought after degree of certainty. -

Is Planck's Constant Ha “Quantum”
Is Planck’s Constant h a “Quantum” Constant? An Alternative Classical Interpretation Timothy H. Boyer Department of Physics, City College of the City University of New York Abstract Although Planck’s constant h is currently regarded as the elementary quantum of action appearing in quantum theory, it can also be interpreted as the multiplicative scale factor setting the scale of classical zero-point radiation appearing in classical electromagnetic theory. Relativistic classical electron theory with classical electromagnetic zero-point radiation gives many results in agreement with quantum theory. The areas of agreement between this classical theory and Nature seem worth further investigation. Introduction Fundamental Constants There are several fundamental constants of nature, including c, e, and h. According to the current physics literature, the constant c is the speed of light in vacuum, e is the charge on the electron, and h is the elementary quantum of action. In this essay, we wish to raise the question as to whether h, Planck’s constant, has been correctly identified. Specifically, we suggest that it may be useful to think of Planck’s constant h as the multiplicative factor setting the scale of classical zero-point radiation, rather than regarding h as associated solely with quantum physics. Planck’s Constant The story behind the introduction of Planck’s constant is well-known and is often sketched out in the first chapters of textbooks of modern physics. Planck first introduced his constant h in 1899 in connection with the spectrum of blackbody radiation. Blackbody radiation is the familiar radiation seen inside a glowing furnace. -

Lecture 18 Fundamentals of Physics Phys 120, Fall 2015 Quantum Physics I
Lecture 18 Fundamentals of Physics Phys 120, Fall 2015 Quantum Physics I A. J. Wagner North Dakota State University, Fargo, ND 58108 Fargo, October 27, 2015 Overview • Origin of Quantum mechanics • Radiation has wave and particle properties • Quantum radiation • Matter has wave and particle properties • Quantum Matter • Nature is non-local and uncertain 1 The Quantum Idea Between 1900 and 1930 Physicists began to realize that many fundamental properties in nature are quantized. Analogy to computation: an analog quantity can take any value, a digital quantity can only take on certain discrete values. If the discretization is small enough, we may not notice the difference. Think of a videogame that shows a rotating objects. The computer, by its digital nature, can only show the object at certain discrete angles, but you will likely not be able to tell the difference. Similarly macroscopic quantities look like they can take on any value or position, but if you look at objects that are small enough this is no longer true. WARNING:This subject is another brain teaser where your intuition is about to fail you. 2 Example: the swing Suppose you sit on a swing at a playground. If you stop pumping you will grad- ually slow down, and the height of your swinging will (appear to) continuously decrease. At the same time you will (appear to) continuously loose energy to friction. If you were much smaller (maybe the size of an atom) you would notice a strange effect: you could only swing with certain discrete amplitudes and you would suddenly switch from one allowed amplitude to another and release a corresponding quantum of energy. -

Physical Interpretation of the Planck's Constant Based on the Maxwell Theory
Physical interpretation of the Planck’s constant based on the Maxwell theory Donald C. Chang Macro-Science Group, Division of LIFS Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong Email: [email protected] Abstract. The discovery of the Planck’s relation is generally regarded as the starting point of quantum physics. The Planck’s constant h is now regarded as one of the most important universal constants. The physical nature of h, however, has not been well understood. It was originally suggested as a fitting constant to explain the black-body radiation. Although Planck had proposed a theoretical justification of h, he was never satisfied with that. To solve this outstanding problem, we used the Maxwell theory to directly calculate the energy and momentum of a radiation wave packet. We found the energy of the wave packet is indeed proportional to its oscillation frequency. This allows us to derive the value of the Planck’s constant. Furthermore, we showed that the emission and transmission of a photon follows the principle of all-or-none. The “strength” of the wave packet can be characterized by , which represents the integrated strength of the vector potential along a transverse axis. We reasoned that should have a fixed cut-off value for all photons. Our results suggest that a wave packet can behave like a particle. This offers a simple explanation to the recent satellite observations that the cosmic microwave background follows closely the black-body radiation as predicted by the Planck’s law. PACS: 03.50.De; 03.65.-w; 03.65.Ta 1. -
Planck's Constant Report
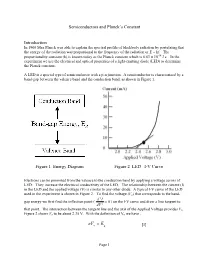
Semiconductors and Planck’s Constant Introduction In 1900 Max Planck was able to explain the spectral profile of blackbody radiation by postulating that the energy of the radiation was proportional to the frequency of the radiation or E = hf. The proportionality constant (h) is known today as the Planck constant which is 6.63 x 10-34 Js. In the experiment we use the electrical and optical properties of a light-emitting diode (LED) to determine the Planck constant. A LED is a special type of semiconductor with a p-n junction. A semiconductor is characterized by a band-gap between the valence band and the conduction band, as shown in Figure 1. Figure 1 Energy Diagram Figure 2 LED I-V Curve Electrons can be promoted from the valence to the conduction band by applying a voltage across of LED. They increase the electrical conductivity of the LED. The relationship between the current (I) in the LED and the applied voltage (V) is similar to any other diode. A typical I-V curve of the LED used in the experiment is shown in Figure 2. To find the voltage (Vo) that corresponds to the band- d 2 I gap energy we first find the inflection point ( 0 ) on the I-V curve and draw a line tangent to dV 2 that point. The intersection between the tangent line and the axis of the Applied Voltage provides Vo. Figure 2 shows Vo to be about 2.35 V. With the definition of Vo we have , eVog E [1] Page 1 where e is electronic charge and Eg is band-gap energy of the LED.