Spatial Genetic Structure of Simarouba Amara Aubl. (Simaroubaceae), a Dioecious, Animal-Dispersed Neotropical Tree, on Barro Colorado Island, Panama
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Agroforestry As a Modern Science
Chapter 2 Evolution of Agroforestry as a Modern Science Jagdish C. Dagar and Vindhya P. Tewari Abstract Agroforestry is as old as agriculture itself. Many of the anecdotal agro- forestry practices, which are time tested and evolved through traditional indigenous knowledge, are still being followed in different agroecological zones. The tradi- tional knowledge and the underlying ecological principles concerning indigenous agroforestry systems around the world have been successfully used in designing the improved systems. Many of them such as improved fallows, homegardens, and park systems have evolved as modern agroforestry systems. During past four decades, agroforestry has come of age and begun to attract the attention of the international scientific community, primarily as a means for sustaining agricultural productivity in marginal lands and solving the second-generation problems such as secondary salinization due to waterlogging and contamination of water resources due to the use of excess nitrogen fertilizers and pesticides. Research efforts have shown that most of the degraded areas including saline, waterlogged, and perturbation ecolo- gies like mine spoils and coastal degraded mangrove areas can be made productive by adopting suitable agroforestry techniques involving highly remunerative compo- nents such as plantation-based farming systems, high-value medicinal and aromatic plants, livestock, fishery, poultry, forest and fruit trees, and vegetables. New con- cepts such as integrated farming systems and urban and peri-urban agroforestry have emerged. Consequently, the knowledge base of agroforestry is being expanded at a rapid pace as illustrated by the increasing number and quality of scientific pub- lications of various forms on different aspects of agroforestry. It is both a challenge and an opportunity to scientific community working in this interdisciplinary field. -

Tree Height Growth in a Neotropical Rain Forest
Ecology, 82(5), 2001, pp. 1460±1472 q 2001 by the Ecological Society of America GETTING TO THE CANOPY: TREE HEIGHT GROWTH IN A NEOTROPICAL RAIN FOREST DEBORAH A. CLARK1 AND DAVID B. CLARK1 Department of Biology, University of Missouri±St. Louis, 8001 Natural Bridge Road, St. Louis, Missouri 63121-4499, USA Abstract. There is still limited understanding of the processes underlying forest dy- namics in the world's tropical rain forests, ecosystems of disproportionate importance in terms of global biogeochemistry and biodiversity. Particularly poorly documented are the nature and time scale of upward height growth during regeneration by the tree species in these communities. In this study, we assessed long-term height growth through ontogeny for a diverse group of canopy and emergent tree species in a lowland neotropical rain forest (the La Selva Biological Station, northeastern Costa Rica). Species were evaluated based on annual height measurements of large samples of individuals in all postseedling size classes, over a 16-yr period (.11 000 increments). The study species were seven nonpi- oneers (Minquartia guianensis, Lecythis ampla, Hymenolobium mesoamericanum, Sima- rouba amara, Dipteryx panamensis, Balizia elegans, and Hyeronima alchorneoides) and two pioneers (Cecropia obtusifolia and Cecropia insignis). For each species, inherent height growth capacity was estimated as the mean of the ®ve largest annual height increments (from different individuals) in each juvenile size class (from 50 cm tall to 20 cm in diameter). All species showed marked ontogenetic increases in this measure of height growth potential. At all sizes, there were highly signi®cant differences among species in height growth potential. -

How Rare Is Too Rare to Harvest? Management Challenges Posed by Timber Species Occurring at Low Densities in the Brazilian Amazon
Forest Ecology and Management 256 (2008) 1443–1457 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco How rare is too rare to harvest? Management challenges posed by timber species occurring at low densities in the Brazilian Amazon Mark Schulze a,b,c,*, James Grogan b,d, R. Matthew Landis e, Edson Vidal b,f a School of Forest Resources and Conservation, University of Florida, P.O. Box 110760, Gainesville, FL 32611, USA b Instituto do Homem e Meio Ambiente da Amazoˆnia (IMAZON), R. Domingos Marreiros, no. 2020 Bairro Fa´tima, Bele´m, Para´ 66060-160, Brazil c Instituto Floresta Tropical (IFT), Caixa Postal 13077, Bele´m, Para´ 66040-970, Brazil d Yale University School of Forestry & Environmental Studies, 360 Prospect St., New Haven, CT 06511, USA e Department of Biology, Middlebury College, Middlebury, VT 05753, USA f ESALQ/Universidade de Sa˜o Paulo, Piracicaba, Sa˜o Paulo 13.418-900, Brazil ARTICLE INFO ABSTRACT Article history: Tropical forests are characterized by diverse assemblages of plant and animal species compared to Received 11 September 2007 temperate forests. Corollary to this general rule is that most tree species, whether valued for timber or Received in revised form 28 January 2008 not, occur at low densities (<1 adult tree haÀ1) or may be locally rare. In the Brazilian Amazon, many of Accepted 28 February 2008 the most highly valued timber species occur at extremely low densities yet are intensively harvested with Keywords: little regard for impacts on population structures and dynamics. These include big-leaf mahogany Conservation (Swietenia macrophylla), ipeˆ (Tabebuia serratifolia and Tabebuia impetiginosa), jatoba´ (Hymenaea courbaril), Reduced-impact logging and freijo´ cinza (Cordia goeldiana). -

Simarouba Monograph 3/1/04 Simarouba Amara, Glauca
Simarouba Monograph 3/1/04 Simarouba amara, glauca Family: Simaroubaceae Synonyms: Quassia simarouba, Zwingera amara, Picraena officinalis, Simarouba medicinalis Other Common Names: Aceituno, bitterwood, bois blanc, bois amer, bois frene, bois negresse, caixeta, cajú-rana, cedro blanco daguilla, dysentery bark, gavilan, malacacheta, marubá, marupá, negrito, palo blanco, palo amargo, paradise tree, pitomba, robleceillo, simaba. Overview Botanical Description Simarouba is indigenous to the Amazon rainforest and other tropical areas in Mexico, Cuba, Haita, Jamaica and Central America. It grows up to 20 m high and has a trunk 50 to 80 cm in diameter. It produces bright green leaves 20 to 50 cm in length, small white flowers, and small red fruit. Ethnobotanical Uses In traditional herbal medicine systems the bark, wood and leaves of simarouba have been used for their amoebicide, analgesic, anthelmintic, antibacterial, antidysenteric, antimalarial, antimicrobial, astringent, febrifuge, stomachic, sudorific, tonic and vermifuge properties. The traditional use of graviola has been recorded in herbal medicine systems in the following countries: Guiana,1 Belize,2 Brazil,3-6 Cuba,7,8 French Guyana,9 Haiti10 and Peru.11 Summary of Traditional Uses of Simarouba:12 Bark: Anemia, anorexia, bitter,diarrhea, dysentery, dyspepsia, emmenagogue, fever, hemorrhages, internal bleeding, intestinal worms, malaria, skin sores, sores, stomach and bowel disorders, tonic, wounds. Leaf: Astringent, colitis, diarrhea, digestive, dysentery, emmenogogue,intestinal worms, malaria, skin affections. Root: Diarrhea, dysentery, flatulence, intestinal worms, malaria, stomach pain, tonic. Primary Uses Internal A bark tea is primarily used as the first line of defense for amebic dysentery and diarrhea. It’s also used for viruses.13 External The bark has been traditionally used in herbal medicine systems externally for wounds and skin sores.2,7 © Copyrighted 2004. -

Woody-Tissue Respiration for Simarouba Amara and Minquartia Guianensis, Two Tropical Wet Forest Trees with Different Growth Habits
Oecologia (1994) 100:213-220 9Springer-Verlag 1994 Michael G. Ryan 9Robert M. Hubbard Deborah A. Clark 9Robert L. Sanford, Jr. Woody-tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits Received: 9 February 1994 / Accepted: 26 July 1994 Abstract We measured CO 2 etflux from stems of two maintenance respiration was 54% of the total CO2 efflux tropical wet forest trees, both found in the canopy, but for Simarouba and 82% for Minquartia. For our sam- with very different growth habits. The species were ple, sapwood volume averaged 23% of stem volume Simarouba amara, a fast-growing species associated when weighted by tree size, or 40% with no size weight- with gaps in old-growth forest and abundant in sec- ing. Using these fractions, and a published estimate of ondary forest, and Minquartia guianensis, a slow-grow- aboveground dry-matter production, we estimate the ing species tolerant of low-light conditions in annual cost of woody tissue respiration for primary old-growth forest. Per unit of bole surface, CO2 efflux forest at La Selva to be 220 or 350 gCm-2year -1, averaged 1.24 gmol m -2 s-1 for Simarouba and depending on the assumed sapwood volume. These 0.83gmolm-2s -1 for Minquartia. C02 elttux was costs are estimated to be less than 13% of the gross highly correlated with annual wood production (r 2= production for the forest. 0.65), but only weakly correlated with stem diameter (r 2 = 0.22). We also partitioned the CO2 efttux into the Key words Woody tissue respiration" Maintenance functional components of construction and mainte- respiration 9Tropical wet forest trees 9Carbon balance nance respiration. -
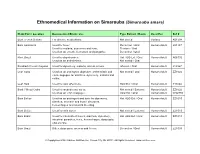
Ethnomedical Information on Simarouba (Simarouba Amara)
Ethnomedical Information on Simarouba (Simarouba amara) Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark French Guiana For diverse medications. Not stated Various K01504 Bark Amazonia Used for fever. Decoction / Oral Human Adult L04137 Used for malaria, dysentery and tonic. Tincture / Oral Used as an emetic, hemostat, and purgative. Decoction / Oral Root Brazil Used to stop diarrhea. Hot H2O Ext / Oral Human Adult A06732 Used as an anthelmintic. Not stated / Oral Rootbark French Guyana Used for dysentery, malaria, and as a tonic. Infusion / Oral Human Adult J12967 Leaf Cuba Used as an astringent, digestive, anthelmintic and Not stated / Oral Human Adult ZZ1022 emmenogogue for diarrhea, dysentery, malaria and colitis. Leaf Haiti Used for skin affections. H2O Ext / Oral Human Adult T13846 Bark / Wood Cuba Used for wounds and sores. Not stated / External Human Adult ZZ1022 Used as an emmenagogue. H2O Ext / Oral Human Adult W02855 Bark Belize Used as an astringent and tonic for dysentery, Hot H2O Ext / Oral Human Adult ZZ1019 diarrhea, stomach and bowel disorders, hemorrhages and internal bleeding. Bark Belize Used for skin sores. Not stated / External Human Adult ZZ1019 Bark Brazil Used for intermittent fevers, diarrhea, dysentery, Hot H2O Ext / Oral Human Adult ZZ1013 intestinal parasites, tonic, hemorrhages, dyspepsia, and anemia. Bark Brazil Bitter, dyspepsia, anemia and fevers. Decoction / Oral Human Adult ZZ1099 © Copyrighted 2004. Raintree Nutrition, Inc. Carson City, NV 89701. All Rights Reserved. www.rain-tree.com Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark + Root Brazil Used for bleeding diarrhea. Decoction / Oral Human Adult ZZ1099 Bark Brazil Used for acute and chronic dysentery, bitter tonic, Infusion / Oral Human Adult ZZ1007 febrifuge, diarrhea, inappetite, and dyspepsia. -

Funtumia Elastica (Preuss) Stapf (Apocynaceae)
Journal of Plant Studies; Vol. 8, No. 2; 2019 ISSN 1927-0461 E-ISSN 1927-047X Published by Canadian Center of Science and Education The Main Ecological Characteristics of a Species Introduced to Martinique in a Dynamic of Invasion: Funtumia Elastica (Preuss) Stapf (Apocynaceae) Philippe Joseph1, Kévine Baillard2, Jean-Philippe Claude2, Yelji Abati2, Séverine Ely-Marius2, Yanis Jean-François2, Stéphane Sophie2, Péguy Major2, José Duranty2, Jean Emile Simphor3 & Jean-Valery Marc3 1Professor, UMR ESPACE DEV-BIORECA, University of the French Antilles, French 2Phd Student, UMR ESPACE DEV-BIORECA, University of the French Antilles, French 3Senior Lecturer, UMR ESPACE DEV-BIORECA, University of the French Antilles, French Correspondence: Philippe Joseph, UMR ESPACE DEV-BIORECA, University of the French Antilles, French. Tel: 596-696-206-141. E-mail: [email protected] Received: March 7, 2019 Accepted: April 4, 2019 Online Published: April 30, 2019 doi:10.5539/jps.v8n2p1 URL: https://doi.org/10.5539/jps.v8n2p1 Abstract Introduced species that become invasive alter the structural and functional organisation of the ecosystems of the host territories because of the absence of certain ecological locks. On a global scale, the consequences are very damaging for many key development-related sectors. Martinique, like all the islands of the Caribbean, is not immune to this phenomenon of biological invasion currently linked to greater globalisation. Among the potentially invasive introduced species and in the light of field observations, Funtumia elastica, native to tropical Africa, appears to have functional traits that could make it a species that is dangerous for local floristic diversity. Since no study exists in Martinique on the ecology of this taxon, we have set up a research protocol based on floristic surveys in various stations marked out by transects subdivided into quadrats. -

Redalyc.Physiochemical Analysis and Centesimal Composition Of
Ciência e Tecnologia de Alimentos ISSN: 0101-2061 [email protected] Sociedade Brasileira de Ciência e Tecnologia de Alimentos Brasil SALES-CAMPOS, Ceci; Medina ARAUJO, Lidia; Teixeira de Almeida MINHONI, Marli; Nogueira de ANDRADE, Meire Cristina Physiochemical analysis and centesimal composition of Pleurotus ostreatus mushroom grown in residues from the Amazon Ciência e Tecnologia de Alimentos, vol. 31, núm. 2, abril-junio, 2011, pp. 456-461 Sociedade Brasileira de Ciência e Tecnologia de Alimentos Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=395940105027 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative ISSN 0101-2061 Ciência e Tecnologia de Alimentos Original Physiochemical analysis and centesimal composition of Pleurotus ostreatus mushroom grown in residues from the Amazon Análise físico-química e composição centecimal do cogumelo Pleurotus ostreatus cultivado em resíduos da Amazônia Ceci SALES-CAMPOS1, Lidia Medina ARAUJO2, Marli Teixeira de Almeida MINHONI3, Meire Cristina Nogueira de ANDRADE3* Abstract The objective of the present work was to evaluate the nutritional composition of mushrooms produced in alternative substrates in agricultural and agro-industrial residues from the Amazon. C, N, pH, moisture, soluble solids, protein, lipids, total fibers, ashes, carbohydrates and energy were determined. Substrates were formulated from Simarouba amara Aubl. and Ochroma piramidale Cav. ex. Lam. Sawdust and from Bactris gasipaes Kunth and Saccharum officinarum stipe. Results showed that the nutritional composition of P. ostreatus varied according to the cultivation substrate and that it can be considered important food due to its nutritional characteristics such as: high protein content; metabolizable carbohydrates and fiber; and low lipids and calories contents. -

Simaroubaceae Family: Botany, Chemical Composition and Biological Activities
Rev Bras Farmacogn 24(2014): 481-501 Review Simaroubaceae family: botany, chemical composition and biological activities Iasmine A.B.S. Alvesa, Henrique M. Mirandab, Luiz A.L. Soaresa,b, Karina P. Randaua,b,* aLaboratório de Farmacognosia, Programa de Pós-graduação em Ciências Farmacêuticas, Universidade Federal de Pernambuco, Recife, PE, Brazil bLaboratório de Farmacognosia, Departamento de Farmácia, Universidade Federal de Pernambuco, Recife, PE, Brazil ARTICLE INFO ABSTRACT Article history: The Simaroubaceae family includes 32 genera and more than 170 species of trees and Received 20 May 2014 brushes of pantropical distribution. The main distribution hot spots are located at tropical Accepted 10 July 2014 areas of America, extending to Africa, Madagascar and regions of Australia bathed by the Pacific. This family is characterized by the presence of quassinoids, secondary metabolites Keywords: responsible of a wide spectrum of biological activities such as antitumor, antimalarial, an- Chemical constituents tiviral, insecticide, feeding deterrent, amebicide, antiparasitic and herbicidal. Although the Simaba chemical and pharmacological potential of Simaroubaceae family as well as its participa- Simarouba tion in official compendia; such as British, German, French and Brazilian pharmacopoeias, Simaroubaceae and patent registration, many of its species have not been studied yet. In order to direct Quassia further investigation to approach detailed botanical, chemical and pharmacological aspects of the Simaroubaceae, the present work reviews -

Antibacterial and Antidiarrheal Activity of Simarouba Amara (Aubl.) Bark
Journal of Applied Pharmaceutical Science Vol. 9(05), pp 088-096 May, 2019 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2019.90511 ISSN 2231-3354 Antibacterial and antidiarrheal activity of Simarouba amara (Aubl.) bark Hegde Veena1, Nagaraj Navya1, Gowda K Sandesh2, Nayaka Boramuthi Thippeswamy1* 1Department of P.G Studies and Research in Microbiology, Kuvempu University, Karnataka, India. 2Niranthara Scientific Solutions Pvt. Ltd., Bengaluru, India. ARTICLE INFO ABSTRACT Received on: 02/11/2018 The present study was conducted to determine the nutritional elements in Simarouba amara (Aubl.) bark aqueous Accepted on: 16/03/2019 extract (SAAE) by inductively coupled plasma optical emission spectrometry (ICP-OES) and the in vitro Available online: 08/05/2019 antibacterial activity against pathogens enterotoxigenic Escherichia coli, Salmonella typhi, Staphylococcous aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa by agar well diffusion, minimum inhibitory, and bactericidal concentration. Then, antidiarrheal effect was studied on castor oil-induced diarrhea in mice model. Recorded Mg > Fe Key words: > Cu > Zn elements in SAAE invariably found to be effective against Gram-positive and Gram-negative pathogens. MIC, MBC, Effective concentration of bark showed the zone of inhibition against enterotoxigenic E. coli (200 mg/ml), S. typhi castor-oil-induced diarrhea, and S. aureus (300 mg/ml), and P. aeruginosa and K. pneumonia (100 mg/ml). The standard ratio between minimum intestinal motility, charcoal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) was meticulously recorded “one” meal test, antidiarrheal index. against all pathogens, which confirms the bactericidal property. Results in mice model prominently showed that SAAE significantly p( < 0.05) reduced the frequency and number of diarrheal episodes, intestinal fluid accumulation, and intestinal transit time in dose-dependent manner. -

Storage of Simarouba Amara Aubl. Seeds Armazenamento De Sementes De Simarouba Amara Aubl
Bol. Mus. Para. Emílio Goeldi. Cienc. Nat., Belém, v. 16, n. 1, p. 89-95, jan.-abr. 2021 Storage of Simarouba amara Aubl. seeds Armazenamento de sementes de Simarouba amara Aubl. Jiovana Santos Pereira Amorim SantosI | Andrea Vita Reis MendonçaII | Edvania da Silva CarvalhoII | Marcus Dhilermando Hora de SouzaII | Manuela Oliveira de SouzaII IUniversidade Estadual de Feira de Santana. Programa de Pós-Graduação em Recursos Genéticos Vegetais. Feira de Santana, Bahia, Brasil IIUniversidade Federal do Recôncavo da Bahia. Cruz das Almas, Bahia, Brasil Abstract: Prior knowledge about the physiological behavior of seeds under storage conditions allows the use of appropriate techniques to maintain viability. The goal of the present study was to classify the behavior of Simarouba amara seeds regarding their physiological potential during storage. The seeds were obtained from mature fruits, collected from five parent plants located at the Joanes-Ipitanga Environmental Protection Area (EPA) (Simões Filho, Bahia) in January 2018. Two experiments were carried out in a completely randomized design. In the first one, seed desiccation tolerance was evaluated with seven moisture contents: 5%, 7.5%, 10%, 12.5%, 15%, 25% and 35.6% (initial moisture). A germination test was carried out to evaluate the seeds’ vigor. In the second experiment, the seeds were stored with moisture contents of 7.5%, 12.5% and 35.6% at temperatures of 8 °C, 15 °C and 20 °C) for two storage periods, two and four months, and samples were taken to determine moisture content and conduct the germination test. Germination tests were performed in BOD chambers at 30 °C and with 12-hour light photoperiod. -

SIMAROUBA Simarouba Amara All Rights Reserved
Technical Data Report for SIMAROUBA Simarouba amara All rights reserved. No part of this document may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage or retrieval system, without written permission from Sage Press, Inc. This document is not intended to provide medical advice and is sold with the understanding that the publisher and the author are not liable for the misconception or misuse of information provided. The author and Sage Press, Inc. shall have neither liability nor responsibility to any person or entity with respect to any loss, damage, or injury caused or alleged to be caused directly or indirectly by the information contained in this document or the use of any plants mentioned. Readers should not use any of the products discussed in this document without the advice of a medical professional. © Copyright 2002 Sage Press, Inc., P.O. Box 80064, Austin, TX 78708-0064. All rights reserved. For additional copies or information regarding this document or other such products offered, call or write at [email protected] or (512) 506-8282. Simarouba Preprinted from Herbal Secrets of the Rainforest, 2nd edition, by Leslie Taylor Published and copyrighted by Sage Press, Inc., © 2002-2003 Family: Simaroubaceae Genus: Simarouba Species: amara, glauca Synonyms: Quassia simarouba, Zwingera amara, Picraena officinalis, Simarouba medicinalis Common Names: Simarouba, gavilan, negrito, marubá, marupá, dysentery bark, bitterwood, paradise tree, palo blanco, robleceillo, caixeta, daguilla, cedro blanco, cajú-rana, malacacheta, palo amargo, pitomba, bois amer, bois blanc, bois frene, bois negresse, simaba Parts Used: Bark, wood, leaves Simarouba is a medium-sized tree that grows up to 20 m high, with a trunk 50 to 80 cm in diameter.