Level 2 Organic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry Lesson 10 Reactions of Organic Molecules Combusition Reactions Substitution Reactions
Organic Chemistry Lesson 10 Reactions of Organic Molecules Combusition Reactions Substitution Reactions Physical Sciences Grade 12 1 Instructions Lesson 10 (16 April 2020) 1. Read ALL the slides in this document. 2. Homework: Just understand the theory for now. • If you have any questions, post them onto your WhatsApp group and your teacher will attend to them when (s)he has a chance. • Additional Resources: • Summary on pg.132 of your textbook. Physical Sciences Grade 12 2 Types of Reactions of Organic Compounds 1. Esterification: formation of an ester from an alcohol and a carboxylic acid. 2. Combustion Reactions (aka Oxidation): organic molecule + oxygen. 3. Substitution Reactions: one or more atoms are replaced by other atoms. (i) alkanes to alkyl halides; (ii) alcohols to alkyl halides; (iii) alkyl halides to alcohols 4. Addition Reactions: one or more atoms are added. (i) hydrogenation; (ii) halogenation; (iii) hydrohalogenation; (iv) hydration 5. Elimination Reactions: one or more atoms are removed. (i) dehydrohalogenation; (ii) dehydration; (iii) cracking of alkanes Physical Sciences Grade 12 3 1. Esterification • Already discussed (refer to earlier notes). Physical Sciences Grade 12 4 2. Combustion Reactions, i.e. Oxidation • A combustion reaction is the reaction of an organic molecule with oxygen. • Please note: “combustion” does NOT mean “explosion”. All combustion reactions release energy but most do so WITHOUT any explosion. • Alkanes – in the form of fossil fuels – are currently the main source of energy worldwide as their combustion is highly exothermic. • Examples: Burning wood for a braai. Propane combusting in a gas stove. Petrol combusting in a car engine. Physical Sciences Grade 12 5 2. -

Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1
Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1. Alkyl Halides (Haloalkane) 2. Nucleophilic Substitution Reactions (SNX, X=1 or 2) 3. Nucleophiles (Acid-Base Chemistry, pka) 4. Leaving Groups (Acid-Base Chemistry, pka) 5. Kinetics of a Nucleophilic Substitution Reaction: An SN2 Reaction 6. A Mechanism for the SN2 Reaction 7. The Stereochemistry of SN2 Reactions 8. A Mechanism for the SN1 Reaction 9. Carbocations, SN1, E1 10. Stereochemistry of SN1 Reactions 11. Factors Affecting the Rates of SN1 and SN2 Reactions 12. --Eliminations, E1 and E2 13. E2 and E1 mechanisms and product predictions In this chapter we will consider: What groups can be replaced (i.e., substituted) or eliminated The various mechanisms by which such processes occur The conditions that can promote such reactions Alkyl Halides (Haloalkane) An alkyl halide has a halogen atom bonded to an sp3-hybridized (tetrahedral) carbon atom The carbon–chlorine and carbon– bromine bonds are polarized because the halogen is more electronegative than carbon The carbon-iodine bond do not have a per- manent dipole, the bond is easily polarizable Iodine is a good leaving group due to its polarizability, i.e. its ability to stabilize a charge due to its large atomic size Generally, a carbon-halogen bond is polar with a partial positive () charge on the carbon and partial negative () charge on the halogen C X X = Cl, Br, I Different Types of Organic Halides Alkyl halides (haloalkanes) sp3-hybridized Attached to Attached to Attached -

Activity 33. Hydrohalogenation & Hydration of Alkynes
Chem 201, Fall 2010 Activity 33, M Nov 22 Activity 33. Hydrohalogenation & Hydration of Alkynes Alkynes undergo many of the same addition reactions that alkenes do, including additions that require carbocation intermediates. Different energy and geometry changes may be required for additions to an alkyne and an alkene so some surprising outcomes may occur with alkynes. Model 1. Addition of HX Hydrogen halides (HCl, HBr, HI) add to alkynes to make vinyl halides . A carbocation mechanism is followed when the reaction is performed in the dark in a peroxide-free solvent. This addition obeys Markovnikov’s rule. A free-radical-chain mechanism is followed when HBr addition is initiated by organic peroxides. This addition gives an anti-Markonikov product. Critical Thinking Questions 1. Draw the carbocations indicated by the two bouncy curved arrows (below). Based on the observation that the carbocation mechanism yields a Markovnikov product, circle the more stable carbocation. 2. (CI) Draw the free radicals indicated by the two competing pathways (below). Based on the observation that the free-radical mechanism yields an anti-Markovnikov product, circle the more stable free radical. 3. The cations and radicals in CTQ #1 and #2 are called vinyl cations and vinyl radicals , respectively. Based on the regioselectivities reported in Model 1, what effect do alkyl substituents at the charged/radical carbon have on the energies of these species? Is this consistent with, or opposed to, their effect on R 3C+ and R 3C• species? 4. The geometry of a vinyl cation can be anticipated using VSEPR. Count electron domains in the cation below and predict all of the HCC bond angles. -

Classifying Halogenoalkanes Reactions Of
10 Halogenoalkanes H Naming Halogenoalkanes H H H H C H H H H Based on original alkane, with a prefix indicating halogen atom: H C C C H Fluoro for F; Chloro for Cl; Bromo for Br; Iodo for I. H C C C C H Br H H H Cl H H Substituents are listed alphabetically 1-bromopropane 2-chloro-2-methylbutane Classifying Halogenoalkanes Halogenoalkanes can be classified as primary, secondary or tertiary depending on the number of carbon atoms attached to the C-X functional group. H H H H H H H H C H H H H H C C C H H C C C H H C C C C H Br H H H Br H H Cl H H Primary halogenoalkane Secondary halogenoalkane Tertiary halogenoalkane One carbon attached to the Two carbons attached to the Three carbons attached to the carbon atom adjoining the carbon atom adjoining the carbon atom adjoining the halogen halogen halogen Reactions of Halogenoalkanes Halogenoalkanes undergo either substitution or elimination reactions Nucleophilic substitution reactions Substitution: swapping a halogen atom for another atom or groups of atoms - - Nucleophile: electron pair donator e.g. :OH , :NH3, CN :Nu represents any nucleophile – they The Mechanism: We draw (or outline) mechanisms to always have a lone pair and act as show in detail how a reaction proceeds electron pair donators Nu:- The nucleophiles The carbon has a small attack the positive H H H H positive charge because carbon atom of the electronegativity H C C X H C C + X- δ+ δ- Nu difference between the carbon and the halogen H H H H We use curly arrows in mechanisms (with A curly arrow will always two line heads) to show the movement of start from a of electrons or two electrons lone pair the centre of a bond The rate of these substitution reactions depends on the strength Bond enthalpy / of the C-X bond kJmol-1 The weaker the bond, the easier it is to break and the faster the reaction. -
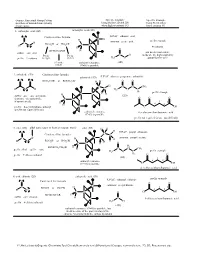
Generic Functional Group Pattern in Ordero of Nomenclature Priority in Our Course. Specific Example Using Suffix (2D and 3D)
Generic Functional Group Pattern Specific Example Specific Example in ordero of nomenclature priority Using Suffix (2D and 3D) Using Prefix when in our course. when highest priority FG lower priority FG 1. carboxylic acid (2D) carboxylic acid (3D) O Condensed line formula IUPAC: ethanoic acid O H H prefix example C H common: acetic acid RCO2H or HO2CR C C R O #-carboxy H O RCH(CO H)R' H suffix: -oic acid 2 O not used in our course FG on FG on C H (acids are the highest priority prefix: #-carboxy the right the left H3C O group that we use) FG in the carbonyl resonance (2D) middle (C=O) is possible 2. anhydride (2D) Condensed line formula anhydride (3D) IUPAC: ethanoic propanoic anhydride O O RCO2COR' or ROCO2CR' H H O O H C C C CH C 3 R O R H3C O C H H 2 prefix example H O C H suffix: -oic -oic anhydride (2D) (just one -oic anhydride, O O O C C O if symmetrical) 3 1 H 4 H 2 prefix: #-acyloxyalkanecarbonyl O O OH (prefix not required for us) carbonyl resonance 4-acyloxymethanebutanoic acid (C=O) is possible (prefix not required for us - too difficult) 3. ester (2D) alkyl name (goes in front as separate word) ester (3D) H H H IUPAC: propyl ethanoate O C Condensed line formula C O C common: propyl acetate C R' H H R O RCO2R' or R'O2CR H H C C H O H H2 RCH(CO2CH3)R' prefix: alkyl suffix: -oate H C C CH3 O H C O C prefix example 3 H prefix: #-alkoxycarbonyl 2 O O (2D) 3 1 carbonyl resonance 4 2 (C=O) is possible O OH 4-methoxycarbonylbutanoic acid 4. -

A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays
ORIGINAL RESEARCH published: 26 April 2021 doi: 10.3389/fchem.2021.682006 A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays Lili Xie 1, Jie Zan 1, Zhijian Yang 1, Qinxia Wu 1, Xiaofeng Chen 1, Xiangyu Ou 1, Caihou Lin 2*, Qiushui Chen 1,3* and Huanghao Yang 1,3* 1 Ministry of Education (MOE) Key Laboratory for Analytical Science of Food Safety and Biology, Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety, College of Chemistry, Fuzhou University, Fuzhou, China, 2 Department of Neurosurgery, Fujian Medical University Union Hospital, Fuzhou, China, 3 Fujian Science and Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, China Detection of haloalkanes is of great industrial and scientific importance because some Edited by: haloalkanes are found serious biological and atmospheric issues. The development Jinyi Lin, of a flexible, wearable sensing device for haloalkane assays is highly desired. Here, Nanjing Tech University, China we develop a paper-based microfluidic sensor to achieve low-cost, high-throughput, Reviewed by: Xiaobao Xu, and convenient detection of haloalkanes using perovskite nanocrystals as a nanoprobe Nanjing University of Science and through anion exchanging. We demonstrate that the CsPbX3 (X = Cl, Br, or I) Technology, China nanocrystals are selectively and sensitively in response to haloalkanes (CH2Cl2, CH2Br2), Xuewen Wang, Northwestern Polytechnical and their concentrations can be determined as a function of photoluminescence University, China spectral shifts of perovskite nanocrystals. In particular, an addition of nucleophilic trialkyl *Correspondence: phosphines (TOP) or a UV-photon-induced electron transfer from CsPbX3 nanocrystals is Caihou Lin [email protected] responsible for achieving fast sensing of haloalkanes. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

UNIT-III: Mechanistic and Sterochemical Aspects of Addition Reaction Involving Electrophiles & Nucleophiles
MCH-204:ORGANIC CHEMISTRY UNIT-III: Mechanistic and sterochemical aspects of addition reaction Involving electrophiles & nucleophiles Prof.Anand Halve S.O.S in Chemistry Jiwaji University Gwalior ELECTROPHILIC ADDITION REACTIONS markovnikov’ additions markovnikov’s rule Addition of hydrogen to an unsymmetrical olefin occurs at those carbon atoms with maximum number of hydrogen atoms. (i.e., the carbon with least substitution). Electronegative group goes to more substituted carbon atom. Such an addition leads to a stabler carbocation. Such a reaction may lead to constitutional isomers but actually one of the products is formed as major product. X X Formed H HX X=F, Cl. X Olefin Not formed origin … X HX X X=F, Cl . carbocation is more stablised in T.S. Stereo specific product H X X carbocation is not enough stablilised in transition state Olefin Consider two possible sites for hydrogen addition (i) terminal or (ii) internal (substituted carbon). The addition of hydrogen at the terminal carbon leads to better stabilization of carbocation, the chances of stabilization increases with increase in conjugation with olefin. The terminal carbocation require higher activation energy which is not a favorable condition, leading to slower reaction rate. However, the generation of non terminal carbocation is assisted by hyperconjugative stabilization leading to a lower activation energy. Alkenes-some facts Due to trigonal planar geometry of olefin carbon atoms the addition can occur on the same side (syn periplanar) or on opposite sides (anti periplanar). Alkenes are generally nucleophilic. The C=C double bond provides a higher energy HOMO (highest occupied molecular orbitals). Electron donating groups increase the rate for electrophilic attack as they assist in carbocation and positive charge stabilization in the TS. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Fully Halogenated Chlorofluorocarbons
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 113 FULLY HALOGENATED CHLOROFLUOROCARBONS This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1990 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. WHO Library Cataloguing in Publication Data Fully halogenated chlorofluorocarbons. (Environmental health criteria ; 113) 1.Freons - adverse effects 2.Freons - toxicity I.Series ISBN 92 4 157113 6 0 (NLM Classification: QV 633) ISSN 0250-863X The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. -
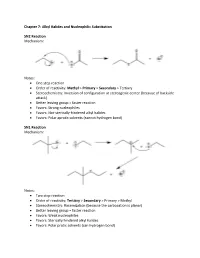
Alkyl Halides and Nucleophilic Substitution SN2 Reaction
Chapter 7: Alkyl Halides and Nucleophilic Substitution SN2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Methyl > Primary > Secondary > Tertiary • Stereochemistry: Inversion of configuration at stereogenic center (because of backside attack) • Better leaving group = faster reaction • Favors: Strong nucleophiles • Favors: Not-sterically-hindered alkyl halides • Favors: Polar aprotic solvents (cannot hydrogen bond) SN1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary > Methyl • Stereochemistry: Racemization (because the carbocation is planar) • Better leaving group = faster reaction • Favors: Weak nucleophiles • Favors: Sterically hindered alkyl halides • Favors: Polar protic solvents (can hydrogen bond) Important Trends Chapter 8: Alkyl Halides and Elimination Reactions E2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: antiperiplanar arrangement of H and X • Better leaving group = faster reaction • Favors: Polar aprotic solvents, strong bases • Products follow Zaitsev rule (more substituted alkene is the major product) E1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: Trigonal planar carbocation intermediate • Better leaving group = faster reaction • Favors: Polar protic solvents, weak bases • Products follow Zaitsev rule Chapter 9: Alcohols, Ethers, and Epoxides Preparation of Alcohols Mechanism: Notes: • SN2 mechanism -

Reaction-Of-Alkenes.Pdf
8 Reactions of Alkenes Goals for Chapter 8 1 Explain why electrophilic additions are among the most common reactions of alkenes. femoral component 2 Predict the products of the reactions of alkenes, including the orientation of the reaction (regio- polyethylene bearing chemistry) and the stereochemistry. tibial plate 3 Propose mechanisms to explain the observed products of alkene reactions. 4 Use retrosynthetic analysis to solve multistep synthesis problems with alkenes as reagents, intermediates, or products. ◀ Polyethylene provides a self-lubricating surface for movement of the metal parts of an artificial knee replacement. The highly cross-linked polyethylene used in these implants is fabricated to be exceptionally tough: It wears only about 0.1 mm per year. Polyethylene is compatible with the human body, and in most cases, it does not cause a foreign body reaction even after years of constant movement in the joint. The alkene double bond is a gateway functional group. Alkene reactions lead to many other functional groups that lay the foundation for the rest of your study of organic chemistry. You can convert alkenes to alkyl halides, epoxides, alcohols, aldehydes, ketones, carboxylic acids, and other functional groups. The reactions of alkenes arise from the reactivity of their carbon–carbon double bonds. Organic chemists enjoy the challenge of taking a simple carbon–carbon double bond and manipulating it in all possible ways to produce other compounds, often mimicking biological reactions that occur in cells. This chapter covers the most common alkene reactions, including their mechanisms, reactivity, orientation, and stereochemistry. Most reactions of alkenes involve addition of atoms or groups across the double bond, with one atom or group adding to each end.