Chapter 7: Haloalkanes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1
Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1. Alkyl Halides (Haloalkane) 2. Nucleophilic Substitution Reactions (SNX, X=1 or 2) 3. Nucleophiles (Acid-Base Chemistry, pka) 4. Leaving Groups (Acid-Base Chemistry, pka) 5. Kinetics of a Nucleophilic Substitution Reaction: An SN2 Reaction 6. A Mechanism for the SN2 Reaction 7. The Stereochemistry of SN2 Reactions 8. A Mechanism for the SN1 Reaction 9. Carbocations, SN1, E1 10. Stereochemistry of SN1 Reactions 11. Factors Affecting the Rates of SN1 and SN2 Reactions 12. --Eliminations, E1 and E2 13. E2 and E1 mechanisms and product predictions In this chapter we will consider: What groups can be replaced (i.e., substituted) or eliminated The various mechanisms by which such processes occur The conditions that can promote such reactions Alkyl Halides (Haloalkane) An alkyl halide has a halogen atom bonded to an sp3-hybridized (tetrahedral) carbon atom The carbon–chlorine and carbon– bromine bonds are polarized because the halogen is more electronegative than carbon The carbon-iodine bond do not have a per- manent dipole, the bond is easily polarizable Iodine is a good leaving group due to its polarizability, i.e. its ability to stabilize a charge due to its large atomic size Generally, a carbon-halogen bond is polar with a partial positive () charge on the carbon and partial negative () charge on the halogen C X X = Cl, Br, I Different Types of Organic Halides Alkyl halides (haloalkanes) sp3-hybridized Attached to Attached to Attached -

Classifying Halogenoalkanes Reactions Of
10 Halogenoalkanes H Naming Halogenoalkanes H H H H C H H H H Based on original alkane, with a prefix indicating halogen atom: H C C C H Fluoro for F; Chloro for Cl; Bromo for Br; Iodo for I. H C C C C H Br H H H Cl H H Substituents are listed alphabetically 1-bromopropane 2-chloro-2-methylbutane Classifying Halogenoalkanes Halogenoalkanes can be classified as primary, secondary or tertiary depending on the number of carbon atoms attached to the C-X functional group. H H H H H H H H C H H H H H C C C H H C C C H H C C C C H Br H H H Br H H Cl H H Primary halogenoalkane Secondary halogenoalkane Tertiary halogenoalkane One carbon attached to the Two carbons attached to the Three carbons attached to the carbon atom adjoining the carbon atom adjoining the carbon atom adjoining the halogen halogen halogen Reactions of Halogenoalkanes Halogenoalkanes undergo either substitution or elimination reactions Nucleophilic substitution reactions Substitution: swapping a halogen atom for another atom or groups of atoms - - Nucleophile: electron pair donator e.g. :OH , :NH3, CN :Nu represents any nucleophile – they The Mechanism: We draw (or outline) mechanisms to always have a lone pair and act as show in detail how a reaction proceeds electron pair donators Nu:- The nucleophiles The carbon has a small attack the positive H H H H positive charge because carbon atom of the electronegativity H C C X H C C + X- δ+ δ- Nu difference between the carbon and the halogen H H H H We use curly arrows in mechanisms (with A curly arrow will always two line heads) to show the movement of start from a of electrons or two electrons lone pair the centre of a bond The rate of these substitution reactions depends on the strength Bond enthalpy / of the C-X bond kJmol-1 The weaker the bond, the easier it is to break and the faster the reaction. -
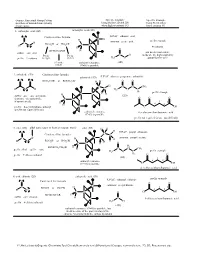
Generic Functional Group Pattern in Ordero of Nomenclature Priority in Our Course. Specific Example Using Suffix (2D and 3D)
Generic Functional Group Pattern Specific Example Specific Example in ordero of nomenclature priority Using Suffix (2D and 3D) Using Prefix when in our course. when highest priority FG lower priority FG 1. carboxylic acid (2D) carboxylic acid (3D) O Condensed line formula IUPAC: ethanoic acid O H H prefix example C H common: acetic acid RCO2H or HO2CR C C R O #-carboxy H O RCH(CO H)R' H suffix: -oic acid 2 O not used in our course FG on FG on C H (acids are the highest priority prefix: #-carboxy the right the left H3C O group that we use) FG in the carbonyl resonance (2D) middle (C=O) is possible 2. anhydride (2D) Condensed line formula anhydride (3D) IUPAC: ethanoic propanoic anhydride O O RCO2COR' or ROCO2CR' H H O O H C C C CH C 3 R O R H3C O C H H 2 prefix example H O C H suffix: -oic -oic anhydride (2D) (just one -oic anhydride, O O O C C O if symmetrical) 3 1 H 4 H 2 prefix: #-acyloxyalkanecarbonyl O O OH (prefix not required for us) carbonyl resonance 4-acyloxymethanebutanoic acid (C=O) is possible (prefix not required for us - too difficult) 3. ester (2D) alkyl name (goes in front as separate word) ester (3D) H H H IUPAC: propyl ethanoate O C Condensed line formula C O C common: propyl acetate C R' H H R O RCO2R' or R'O2CR H H C C H O H H2 RCH(CO2CH3)R' prefix: alkyl suffix: -oate H C C CH3 O H C O C prefix example 3 H prefix: #-alkoxycarbonyl 2 O O (2D) 3 1 carbonyl resonance 4 2 (C=O) is possible O OH 4-methoxycarbonylbutanoic acid 4. -

A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays
ORIGINAL RESEARCH published: 26 April 2021 doi: 10.3389/fchem.2021.682006 A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays Lili Xie 1, Jie Zan 1, Zhijian Yang 1, Qinxia Wu 1, Xiaofeng Chen 1, Xiangyu Ou 1, Caihou Lin 2*, Qiushui Chen 1,3* and Huanghao Yang 1,3* 1 Ministry of Education (MOE) Key Laboratory for Analytical Science of Food Safety and Biology, Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety, College of Chemistry, Fuzhou University, Fuzhou, China, 2 Department of Neurosurgery, Fujian Medical University Union Hospital, Fuzhou, China, 3 Fujian Science and Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, China Detection of haloalkanes is of great industrial and scientific importance because some Edited by: haloalkanes are found serious biological and atmospheric issues. The development Jinyi Lin, of a flexible, wearable sensing device for haloalkane assays is highly desired. Here, Nanjing Tech University, China we develop a paper-based microfluidic sensor to achieve low-cost, high-throughput, Reviewed by: Xiaobao Xu, and convenient detection of haloalkanes using perovskite nanocrystals as a nanoprobe Nanjing University of Science and through anion exchanging. We demonstrate that the CsPbX3 (X = Cl, Br, or I) Technology, China nanocrystals are selectively and sensitively in response to haloalkanes (CH2Cl2, CH2Br2), Xuewen Wang, Northwestern Polytechnical and their concentrations can be determined as a function of photoluminescence University, China spectral shifts of perovskite nanocrystals. In particular, an addition of nucleophilic trialkyl *Correspondence: phosphines (TOP) or a UV-photon-induced electron transfer from CsPbX3 nanocrystals is Caihou Lin [email protected] responsible for achieving fast sensing of haloalkanes. -

Unit 11 Halogen Derivatives
UNIT 11 HALOGEN DERIVATIVES Structure 11.1 Introduction Objectives 11.2 Classification of Halogen Derivatives 11.3 Preparation of Halogen Derivatives Alkyl Halides Aryl Halides Alkenyl Halides 11.4 Structure and Properties of Halogen Derivatives Structure of Halogen Derivatives Physical Properties of Halogen Derivatives Spectral Properties of Halogen Derivatives Chemical Properties of Alkyl Halides Chemical Properties of Aryl and Alkenyl Halides 11.5 Organometallic Compounds 11.6 Polyhalogen Derivatives Dihalogen Derivatives Trihalogen Derivatives 11.7 Uses of Halogen Derivatives 11.8 Lab Detection 11.9 Summary 11.10 Terminal Questions 11.11 Answers 11.1 INTRODUCTION In Block 2, we have described the preparation and reactions of hydrocarbons and some heterocyclic compounds. In this unit and in the next units, we will srudy some derivatives of hydrocarbons. Replacement of one or more hydrogen atoms in a hycimcarbon by halogen atom(s) [F, CI, Br, or I:] gives the halogen derivatives. These compounds are important laboratory and industrial solvents and serve as intermediates in the synthesis of other organic compounds. Many chlorohydrocarbons have acquired importance as insecticides. Although there are not many naturally occurring halogen derivatives yet you might be familiar with one such compound, thyroxin-a thyroid hormone. In this unit, we shall take up the chemistry of the halogen derivatives in detail beginning with classification of halogen derivatives and then going over to methods of their preparation. We shall also discuss the reactivity'of halogen compounds and focus our attention specially, on some important reactions such as nucleophilic substitution (SN)and elimination (E) reactions. Finally, we shall take up uses of halogen derivatives and the methods for their detection. -

Chapter 7 - Alkenes and Alkynes I
Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name of acetylene 7.2 - The (E)-(Z) System for Designating Alkene Diastereomers - To determine E or Z, look at the two groups attached to one carbon atom of the double bond and decide which has higher priority. Then, repeat this at the other carbon atom. This system is not used for cycloalkenes - If the two groups of higher priority are on the same side of the double bond, the alkene is designated Z. If the two groups of higher priority are on opposite sides of the double bond, the alkene is designated E - Hydrogenation is a syn/cis addition 7.3 - Relative Stabilities of Alkenes - The trans isomer is generally more stable than the cis isomer due to electron repulsions - The addition of hydrogen to an alkene, hydrogenation, is exothermic (heat of hydrogenation) - The greater number of attached alkyl groups, the greater the stability of an alkene 7.5 - Synthesis of Alkenes via Elimination Reactions, 7.6 - Dehydrohalogenation of Alkyl Halides How to Favor E2: - Reaction conditions that favor elimination by E1 should be avoided due to the highly competitive SN 1 mechanism - To favor E2, a secondary or tertiary alkyl halide should be used - If there is only a possibility for a primary alkyl halide, use a bulky base - Use a higher concentration of a strong and nonpolarizable base, like an alkoxide - EtONa=EtOH favors the more substituted double bond -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Fully Halogenated Chlorofluorocarbons
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 113 FULLY HALOGENATED CHLOROFLUOROCARBONS This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1990 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. WHO Library Cataloguing in Publication Data Fully halogenated chlorofluorocarbons. (Environmental health criteria ; 113) 1.Freons - adverse effects 2.Freons - toxicity I.Series ISBN 92 4 157113 6 0 (NLM Classification: QV 633) ISSN 0250-863X The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. -

Organic Seminar Abstracts
Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/organicsemin1971752univ / a ORGANIC SEMINAR ABSTRACTS 1971-1972 Semester II School of Chemical Sciences Department of Chemistry- University of Illinois Urbana, Illinois 3 SEMINAR TOPICS II Semester 1971-72 Reactions of Alkyl Ethers Involving n- Complexes on a Reaction Pathway 125 Jerome T. Adams New Syntheses of Helicenes 127 Alan Morrice Recent Advances in Drug Detection and Analysis I36 Ronald J. Convers The Structure of Carbonium Ions with Multiple Neighboring Groups 138 William J. Work Recent Reactions of the DMSO-DCC Reagent ll+O James A. Franz Nucleophilic Acylation 1^2 Janet Ollinger The Chemistry of Camptothecin lU^ Dale Pigott Stereoselective Syntheses and Absolute Configuration of Cecropia Juvenile Horomone 1U6 John C. Greenfield Uleine and Related Aspidosperma Alkaloids 155 Glen Tolman Strategies in Oligonucleotide Synthesis 162 Graham Walker Stable Carbocations: C-13 Nuclear Magnetic Resonance Studies 16U Moses W. McMillan Organic Chlorosulfinates 166 Steven W. Moje Recent Advances in the Chemistry of Penicillins and Cephalosporins 168 Ronald Stroshane Cerium (iv) Oxidations 175 William C. Christopfel A New Total Synthesis of Vitamin D 18 William Graham Ketone Transpositions 190 Ann L. Crumrine - 125 - REACTIONS OF ALKYL ETHERS INVOLVING n-COMPLEXES ON A REACTION PATHWAY Reported by Jerome T. Adams February 2k 1972 The n-complex (l) has been described as an intermediate on the reaction pathway for electrophilic aromatic substitution and acid catalyzed rearrange- ment of alkyl aryl ethers along with sigma (2) and pi (3) type intermediates. 1 ' 2 +xR Physical evidence for the existence of n-complexes of alkyl aryl ethers was found in the observation of methyl phenyl ononium ions by nmr 3 and ir observation of n-complexes of anisole with phenol. -

Elimination Reactions Are Described
Introduction In this module, different types of elimination reactions are described. From a practical standpoint, elimination reactions widely used for the generation of double and triple bonds in compounds from a saturated precursor molecule. The presence of a good leaving group is a prerequisite in most elimination reactions. Traditional classification of elimination reactions, in terms of the molecularity of the reaction is employed. How the changes in the nature of the substrate as well as reaction conditions affect the mechanism of elimination are subsequently discussed. The stereochemical requirements for elimination in a given substrate and its consequence in the product stereochemistry is emphasized. ELIMINATION REACTIONS Objective and Outline beta-eliminations E1, E2 and E1cB mechanisms Stereochemical considerations of these reactions Examples of E1, E2 and E1cB reactions Alpha eliminations and generation of carbene I. Basics Elimination reactions involve the loss of fragments or groups from a molecule to generate multiple bonds. A generalized equation is shown below for 1,2-elimination wherein the X and Y from two adjacent carbon atoms are removed, elimination C C C C -XY X Y Three major types of elimination reactions are: α-elimination: two atoms or groups are removed from the same atom. It is also known as 1,1-elimination. H R R C X C + HX R Both H and X are removed from carbon atom here R Carbene β-elimination: loss of atoms or groups on adjacent atoms. It is also H H known as 1,2- elimination. R C C R R HC CH R X H γ-elimination: loss of atoms or groups from the 1st and 3rd positions as shown below. -

Organometallics in Regio Enantio-Selective Synthes
THESIS IN JOINT SUPE RVISION DOTTORATO IN SCIENZE CHIMICHE DOCTORAT DE L’UNIVERSITE DE ROUEN SETTORE DISCIPLINARE CHIMICA ORGANICA CHIM/06 Spécialité: CHIMIE XXII CICLO Discipline: CHIMIE ORGANIQUE Organometallics in Regio --- aaandand EnantioEnantio----Selective Synthesis: Structures and Reactivity Gabriella Barozzino Consiglio members of jury Prof. Giovanni POLI (Rapporteur) Université Pierre et Marie Curie (Paris VI) Prof. Paolo VENTURELLO (Rapporteur) Università di Torino Prof. Annie-Claude GAUMONT (Examin er) Université de Caen Prof. Vito CAPRIATI (Examiner) Università di Bari Prof. Hassan OULYADI (Directeur de Thèse à Rouen) Université de Rouen Dr. Alessandro MORDINI (Direttore di Tesi a Firenze) Università di Firenze 2007-2009 INDEX Abbreviations and Acronyms vii Abstracts 1 1. General Introduction 3 1.1. Introduction 3 1.2. Aim of the project 5 1.3. Organolithium chemistry 7 1.3.1. Organolithium structures 7 1.3.2. Organolithium aggregates 8 1.3.3. Dynamic of organolithium compounds 10 1.3.4. Analysis of organolithium compounds 12 First Part 2. Introduction to the Base-Promoted Rearrangement of Strained Heterocycles 15 2.1. Superbases 15 2.1.1. Structures and reactivity 16 2.1.2. Applications 18 2.2. Epoxides and aziridines 20 2.2.1. Epoxides 21 2.2.2. Aziridines 22 2.3. Previous results about the base-induced isomerizations of epoxides 23 2.4. Previous results about the base-induced isomerizations of epoxy ethers 26 2.5. Previous results about the base-induced isomerizations of aziridines 30 2.5.1. Isomerizations of aziridines 31 2.5.2. Elaboration of allylic amines 34 i Index 3. Rearrangements of Aziridinyl Ethers Mediated by Superbases 37 3.1.