Activity 33. Hydrohalogenation & Hydration of Alkynes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry Lesson 10 Reactions of Organic Molecules Combusition Reactions Substitution Reactions
Organic Chemistry Lesson 10 Reactions of Organic Molecules Combusition Reactions Substitution Reactions Physical Sciences Grade 12 1 Instructions Lesson 10 (16 April 2020) 1. Read ALL the slides in this document. 2. Homework: Just understand the theory for now. • If you have any questions, post them onto your WhatsApp group and your teacher will attend to them when (s)he has a chance. • Additional Resources: • Summary on pg.132 of your textbook. Physical Sciences Grade 12 2 Types of Reactions of Organic Compounds 1. Esterification: formation of an ester from an alcohol and a carboxylic acid. 2. Combustion Reactions (aka Oxidation): organic molecule + oxygen. 3. Substitution Reactions: one or more atoms are replaced by other atoms. (i) alkanes to alkyl halides; (ii) alcohols to alkyl halides; (iii) alkyl halides to alcohols 4. Addition Reactions: one or more atoms are added. (i) hydrogenation; (ii) halogenation; (iii) hydrohalogenation; (iv) hydration 5. Elimination Reactions: one or more atoms are removed. (i) dehydrohalogenation; (ii) dehydration; (iii) cracking of alkanes Physical Sciences Grade 12 3 1. Esterification • Already discussed (refer to earlier notes). Physical Sciences Grade 12 4 2. Combustion Reactions, i.e. Oxidation • A combustion reaction is the reaction of an organic molecule with oxygen. • Please note: “combustion” does NOT mean “explosion”. All combustion reactions release energy but most do so WITHOUT any explosion. • Alkanes – in the form of fossil fuels – are currently the main source of energy worldwide as their combustion is highly exothermic. • Examples: Burning wood for a braai. Propane combusting in a gas stove. Petrol combusting in a car engine. Physical Sciences Grade 12 5 2. -

A Palladium-Catalyzed Approach to Allenic Aromatic Ethers and First Total
Chemical Science EDGE ARTICLE View Article Online View Journal | View Issue A palladium-catalyzed approach to allenic aromatic ethers and first total synthesis of terricollene A† Cite this: Chem. Sci.,2021,12,9347 a b b b a a a All publication charges for this article Chaofan Huang, Fuchun Shi, Yifan Cui,‡ Can Li,‡ Jie Lin,‡ Qi Liu,‡ Anni Qin,‡ have been paid for by the Royal Society Huanan Wang,‡a Guolin Wu,‡a Penglin Wu,‡a Junzhe Xiao, ‡b Haibo Xu,‡b of Chemistry Yuan Yuan,‡a Yizhan Zhai,‡b Wei-Feng Zheng,‡a Yangguangyan Zheng, ‡a Biao Yu*b and Shengming Ma *ab A palladium-catalyzed C–O bond formation reaction between phenols and allenylic carbonates to give 2,3- allenic aromatic ethers with decent to excellent yields under mild reaction conditions has been described. A Received 7th April 2021 variety of synthetically useful functional groups are tolerated and the synthetic utility of this method has Accepted 4th June 2021 been demonstrated through a series of transformations of the allene moiety. By applying this reaction as DOI: 10.1039/d1sc01896e the key step, the total syntheses of naturally occurring allenic aromatic ethers, eucalyptene and rsc.li/chemical-science terricollene A (first synthesis; 4.5 g gram scale), have been accomplished. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Introduction Results and discussion Aromatic ethers are prevalent and prominent in a variety of In contrast to the well-established metal-catalyzed coupling natural products, pharmaceuticals, and agrochemicals.1 Tradi- reactions between aryl halides and phenols or aliphatic alcohols tional methods for the preparation of aromatic ethers include (Scheme 1b),9 metal-catalyzed coupling reactions of aryl halides the Williamson ether synthesis,2 direct nucleophilic substitu- tion reactions,3 Mitsunobu reaction,4 Ullman-type couplings of alkoxides with aryl halides,5 etc. -

Kinetics and Products Distribution of Selective Catalytic Hydration of Ethylene and Propylene Oxides in Concentrated Aqueous Solutions
Organic Process Research & Development 2002, 6, 660-664 Kinetics and Products Distribution of Selective Catalytic Hydration of Ethylene and Propylene Oxides in Concentrated Aqueous Solutions I. A. Kozlovsky, R. A. Kozlovsky,* A. V. Koustov, M. G. Makarov, J. P. Suchkov, and V. F. Shvets D.I.Mendeleev University of Chemical Technology of Russia, Chair of Basic Organic and Petrochemical Synthesis, 9 Miusskaya Square, Moscow 125047, Russia Abstract: and metalate anions.I" The kinetics and reaction mechanism The kinetics of selective hydration of ethylene- and propylene of the hydration of a-oxides using homogeneous catalysis oxides in concentrated aqueous solutions is studied during by salts have been explicitly studied.3,IO,1l The kinetic data homogeneous catalysis by sodium bicarbonate. The mathemati obtained have shown that at a concentration of some salts cal model of the process with determined parameters adequately of about 0.5 molJL the distribution factor b = klko is reduced describing the rate of the reaction and products distribution is lO-fold more (to 0.1-0.2). This enables the production of developed. monoglycol with high selectivity at water/oxide molar ratios close to 1. We have used hereinafter homogeneous nucleo philic catalysts with the above-mentioned properties for the Introduction creation of industrial heterogeneous catalysts of a selective The reaction of ethylene- and propylene oxides hydration hydration of ethylene- and propylene oxides by means of is an industrial way of obtaining of glycols, in particular an immobilization of anions of salts on heterogeneous ethylene glycol-one of the most-produced large-scale carriers. 12-17 The largest ethylene glycol producers Shell, 18-24 products of industrial organic synthesis, with the world (4) Masuda, T. -

Vapor Phase Hydration of Ethylene Catalyzed by Solid Acids*
47 Vapor Phase Hydration of Ethylene Catalyzed by Solid Acids* by Kozo Tanabe** and Masahiro Nitta** Summary: The hydration of ethyleneover various solid acids such as metal sulfates, phos- phates, oxides, solid phosphoricacid and cation exchangedzeolites was investigatedin a closed circulation system at 160~300℃. Ferric and aluminum sulfates and zeolites Y showed high catalytic activity in comparison with solid phosphoricacid, SiO2-Al2O3,zeolites A and all other catalysts. The selectivityfor ethanolformation was extremelyhigh in metal sulfates and zeolites A, but low in SiO2-Al2O3 and zeolites Y. The order of activities of metal sulfates was Fe2(SO4)3>Al2(SO4)3>Ni- SO4> Cr2(SO4)3>CuSO4>MnSO4>CaSO4. The catalysts those acid strength in their dried state was as strong as -8.2<Ho≦-3 were found to be active and selective for ethanol for- mation. The activitiesof cation exchangedzeolites A which varied in order of Mg-A>Cd-A> Zn-A>Ca-A>Ag-A>Sr-A>La-A~Ce-A were shown to correlate with the electronegativities of exchangedcations and the adsorptionheats of ethylene. The rate of ethanolformation increasedas the mole ratio of H2O/C2H4 decreased. The experimenton the reaction of ethylenewith heavy water showed that deuterium did not transfer into ethyleneat all. It was concludedby analyzing the kinetic data that theformation of an ad- sorbedethyl carbonium ion from an adsorbedethylene and a proton on acid sites was the rate-deter- miningstep of the hydration. 1 Introduction 2 Experimental The direct synthesis of alcohol from ethylene 2.1 Catalysts -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

UNIT-III: Mechanistic and Sterochemical Aspects of Addition Reaction Involving Electrophiles & Nucleophiles
MCH-204:ORGANIC CHEMISTRY UNIT-III: Mechanistic and sterochemical aspects of addition reaction Involving electrophiles & nucleophiles Prof.Anand Halve S.O.S in Chemistry Jiwaji University Gwalior ELECTROPHILIC ADDITION REACTIONS markovnikov’ additions markovnikov’s rule Addition of hydrogen to an unsymmetrical olefin occurs at those carbon atoms with maximum number of hydrogen atoms. (i.e., the carbon with least substitution). Electronegative group goes to more substituted carbon atom. Such an addition leads to a stabler carbocation. Such a reaction may lead to constitutional isomers but actually one of the products is formed as major product. X X Formed H HX X=F, Cl. X Olefin Not formed origin … X HX X X=F, Cl . carbocation is more stablised in T.S. Stereo specific product H X X carbocation is not enough stablilised in transition state Olefin Consider two possible sites for hydrogen addition (i) terminal or (ii) internal (substituted carbon). The addition of hydrogen at the terminal carbon leads to better stabilization of carbocation, the chances of stabilization increases with increase in conjugation with olefin. The terminal carbocation require higher activation energy which is not a favorable condition, leading to slower reaction rate. However, the generation of non terminal carbocation is assisted by hyperconjugative stabilization leading to a lower activation energy. Alkenes-some facts Due to trigonal planar geometry of olefin carbon atoms the addition can occur on the same side (syn periplanar) or on opposite sides (anti periplanar). Alkenes are generally nucleophilic. The C=C double bond provides a higher energy HOMO (highest occupied molecular orbitals). Electron donating groups increase the rate for electrophilic attack as they assist in carbocation and positive charge stabilization in the TS. -

Organic Chemistry Peer Tutoring Department CHEM 51B University
Organic Chemistry Peer Tutoring Department CHEM 51B University of California, Irvine Professor Pronin Aubrey Taduran ([email protected]) http://sites.uci.edu/ochemtutors Crystal Wong ([email protected]) Georgene Vasquez ([email protected]) Midterm 2 Review Packet Key 1. Draw the starting reagents for the first reaction and the product(s) for the second reaction. Determine the mechanism performed in each reaction. Explain how the second mechanism was determined. Alcohol dehydration can be determined from the strong acidic reagent (H2SO4). Because the produced alcohol is a 2° alcohol, we can conclude that the dehydration reaction underwent an E1 mechanism. 2. Predict the product(s) for the following reaction. Indicate the major and minor product if applicable. The alcohol molecule has two differently positioned beta hydrogens that allow the dehydration of alcohols in acid. The two differently positioned beta hydrogens result in two different products. 3. Rank the following alcohol molecules from fastest to slowest (1-fastest, 3-slowest) rate of reaction with HCl. Determine mechanism each alcohol would undergo when reacting to HCl and the common byproduct that will result from the reaction. The common byproduct created through the conversion of alcohols to alkyl halides with HX is H2O. 4. Provide the product and mechanism of the following reaction. 5. Provide the product and mechanism of the following reaction. 6. Complete the table below for converting alcohols to alkyl halides. HX HX PBr SOCl 3 2 Alcohol (CH3OH, 1°, 2°, 3°) CH3OH, 1° 2°, 3° CH3OH, 1°, 2° CH3OH, 1°, 2° Mechanism (SN1, SN2) SN2 SN1 SN2 SN2 7. Determine the starting reagents for the following reaction. -
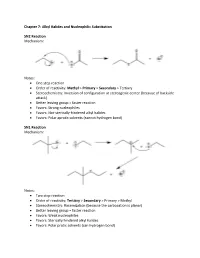
Alkyl Halides and Nucleophilic Substitution SN2 Reaction
Chapter 7: Alkyl Halides and Nucleophilic Substitution SN2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Methyl > Primary > Secondary > Tertiary • Stereochemistry: Inversion of configuration at stereogenic center (because of backside attack) • Better leaving group = faster reaction • Favors: Strong nucleophiles • Favors: Not-sterically-hindered alkyl halides • Favors: Polar aprotic solvents (cannot hydrogen bond) SN1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary > Methyl • Stereochemistry: Racemization (because the carbocation is planar) • Better leaving group = faster reaction • Favors: Weak nucleophiles • Favors: Sterically hindered alkyl halides • Favors: Polar protic solvents (can hydrogen bond) Important Trends Chapter 8: Alkyl Halides and Elimination Reactions E2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: antiperiplanar arrangement of H and X • Better leaving group = faster reaction • Favors: Polar aprotic solvents, strong bases • Products follow Zaitsev rule (more substituted alkene is the major product) E1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: Trigonal planar carbocation intermediate • Better leaving group = faster reaction • Favors: Polar protic solvents, weak bases • Products follow Zaitsev rule Chapter 9: Alcohols, Ethers, and Epoxides Preparation of Alcohols Mechanism: Notes: • SN2 mechanism -

Reaction-Of-Alkenes.Pdf
8 Reactions of Alkenes Goals for Chapter 8 1 Explain why electrophilic additions are among the most common reactions of alkenes. femoral component 2 Predict the products of the reactions of alkenes, including the orientation of the reaction (regio- polyethylene bearing chemistry) and the stereochemistry. tibial plate 3 Propose mechanisms to explain the observed products of alkene reactions. 4 Use retrosynthetic analysis to solve multistep synthesis problems with alkenes as reagents, intermediates, or products. ◀ Polyethylene provides a self-lubricating surface for movement of the metal parts of an artificial knee replacement. The highly cross-linked polyethylene used in these implants is fabricated to be exceptionally tough: It wears only about 0.1 mm per year. Polyethylene is compatible with the human body, and in most cases, it does not cause a foreign body reaction even after years of constant movement in the joint. The alkene double bond is a gateway functional group. Alkene reactions lead to many other functional groups that lay the foundation for the rest of your study of organic chemistry. You can convert alkenes to alkyl halides, epoxides, alcohols, aldehydes, ketones, carboxylic acids, and other functional groups. The reactions of alkenes arise from the reactivity of their carbon–carbon double bonds. Organic chemists enjoy the challenge of taking a simple carbon–carbon double bond and manipulating it in all possible ways to produce other compounds, often mimicking biological reactions that occur in cells. This chapter covers the most common alkene reactions, including their mechanisms, reactivity, orientation, and stereochemistry. Most reactions of alkenes involve addition of atoms or groups across the double bond, with one atom or group adding to each end. -

Hydrohalogenation
Hydrohalogenation A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.[1][2][3] If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with fewer hydrogen substituents, an observation known as Markovnikov's rule. This is due to the abstraction of a hydrogen atom by the alkene from the acid (HX) to form the most stable carbocation (relative stability: 3°>2°>1°>methyl), as well as generating a halogen anion. A simple example of a hydrochlorination is that of indene with hydrogen chloride gas (no solvent):[4] Alkynes also undergo hydrohalogenation reactions. Depending on the exact substrate, alkyne hydrohalogenation can proceed though a concerted protonation/nucleophilic attack (AdE3) or stepwise by first - protonating the alkyne to form a vinyl cation, followed by attack of HX/X to give the product (AdE2) (see electrophile for arrow pushing).[5] As in the case of alkenes, the regioselectivity is determined by the relative ability of the carbon atoms to stabilize positive charge (either a partial charge in the case of a concerted transition state or a full formal charge for a discrete vinyl cation). Depending on reaction conditions, the main product could be this initially formed alkenyl halide, or the product of twice hydrohalogenation to form a dihaloalkane. In most cases, the main regioisomer formed is the gem-dihaloalkane.[6] This regioselectivity is rationalized by the resonance stabilization of a neighboring carbocation by a lone pair on the initially installed halogen. -

On the Michael Addition of Water to ,Unsaturated Ketones Using Amino
SHORT COMMUNICATION DOI: 10.1002/ejoc.201301230 On the Michael Addition of Water to α,β-Unsaturated Ketones Using Amino Acids Verena Resch,*[a] Christiane Seidler,[a] Bi-Shuang Chen,[a] Ian Degeling,[a] and Ulf Hanefeld[a] Keywords: Organocatalysis / Amino acids / Water chemistry / Green chemistry / Michael addition The use of water as a nucleophile for Michael additions is as the best candidate. To obtain a better insight and to deter- still a challenge in organic chemistry. In this report we de- mine the minimum requirements of the catalyst, several scribe the use of amino acids as catalysts for the Michael ad- structurally related compounds were tested. The reaction dition of water to α,β-unsaturated ketones. All 20 proteino- was characterized in terms of conditions and equilibrium. genic amino acids were screened and L-lysine was identified Introduction However, most of the methods furnishing racemic products suffer from complex, cumbersome or expensive preparation The Michael addition of water to α,β-unsaturated of the catalysts. ketones still seems to be a difficult task to achieve by using In the last few years, amino acids have played an impor- chemical methods. Although in nature this reaction is ubiq- tant role in organocatalysis. In particular, proline and its uitous due to its presence in many metabolic cycles, only derivatives have been established as versatile catalysts for a a few methods using non-enzymatic approaches have been variety of different reactions including Michael additions reported. Apart from enzymatic methods using hy- (for selected examples see ref.[9] and for reviews see ref.[10] dratases,[1] for example, fumarase, malease, citraconase and and references cited therein). -

Improved Processing Isoolefin Polymers Verarbeitung Von Isoolefin-Polymeren Transformation De Polymeres D'iso-Olefine
Europaisches Patentamt (19) European Patent Office Office europeenopeen des brevets EP 0320 263 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) lntCI.6: C08L 23/22 of the grant of the patent: 06.03.1996 Bulletin 1996/10 (21) Application number: 88311634.5 (22) Date of filing: 08.12.1988 (54) Improved processing isoolefin polymers Verarbeitung von Isoolefin-Polymeren Transformation de polymeres d'iso-olefine (84) Designated Contracting States: • Wang, Hsien-Chang BE DE ES FR GB IT NL SE Edison New Jersey 08820 (US) • Handy, Debra Clark (30) Priority: 11.12.1987 US 131938 Basking Ridge New Jersey 07920 (US) • Fusco, James Vincent (43) Date of publication of application: Red Bank New Jersey 07701 (US) 14.06.1989 Bulletin 1989/24 (74) Representative: Darby, David Thomas et al (60) Divisional application: 95110803.4 Abel & Imray Northumberland House (73) Proprietor: EXXON CHEMICAL PATENTS INC. 303-306 High Holborn Florham Park New Jersey 07932 (US) London WC1V7LH (GB) (72) Inventors: (56) References cited: • Powers, Kenneth William EP-A- 0 117 635 EP-A- 0 234 114 Berkeley Heights New Jersey 07922 (US) US- A- 4 139 695 US-A- 4 350 621 DO CO CO CM o CM CO Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give o notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid.