The Scfβ-Trcp E3 Ubiquitin Ligase Regulates Immune Receptor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PARSANA-DISSERTATION-2020.Pdf
DECIPHERING TRANSCRIPTIONAL PATTERNS OF GENE REGULATION: A COMPUTATIONAL APPROACH by Princy Parsana A dissertation submitted to The Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland July, 2020 © 2020 Princy Parsana All rights reserved Abstract With rapid advancements in sequencing technology, we now have the ability to sequence the entire human genome, and to quantify expression of tens of thousands of genes from hundreds of individuals. This provides an extraordinary opportunity to learn phenotype relevant genomic patterns that can improve our understanding of molecular and cellular processes underlying a trait. The high dimensional nature of genomic data presents a range of computational and statistical challenges. This dissertation presents a compilation of projects that were driven by the motivation to efficiently capture gene regulatory patterns in the human transcriptome, while addressing statistical and computational challenges that accompany this data. We attempt to address two major difficulties in this domain: a) artifacts and noise in transcriptomic data, andb) limited statistical power. First, we present our work on investigating the effect of artifactual variation in gene expression data and its impact on trans-eQTL discovery. Here we performed an in-depth analysis of diverse pre-recorded covariates and latent confounders to understand their contribution to heterogeneity in gene expression measurements. Next, we discovered 673 trans-eQTLs across 16 human tissues using v6 data from the Genotype Tissue Expression (GTEx) project. Finally, we characterized two trait-associated trans-eQTLs; one in Skeletal Muscle and another in Thyroid. Second, we present a principal component based residualization method to correct gene expression measurements prior to reconstruction of co-expression networks. -

By Submitted in Partial Satisfaction of the Requirements for Degree of in In
Developments of Two Imaging based Technologies for Cell Biology Researches by Xiaowei Yan DISSERTATION Submitted in partial satisfaction of the requirements for degree of DOCTOR OF PHILOSOPHY in Biochemistry and Molecular Biology in the GRADUATE DIVISION of the UNIVERSITY OF CALIFORNIA, SAN FRANCISCO Approved: ______________________________________________________________________________Ronald Vale Chair ______________________________________________________________________________Jonathan Weissman ______________________________________________________________________________Orion Weiner ______________________________________________________________________________ ______________________________________________________________________________ Committee Members Copyright 2021 By Xiaowei Yan ii DEDICATION Everything happens for the best. To my family, who supported me with all their love. iii ACKNOWLEDGEMENTS The greatest joy of my PhD has been joining UCSF, working and learning with such a fantastic group of scientists. I am extremely grateful for all the support and mentorship I received and would like to thank: My mentor, Ron Vale, who is such a great and generous person. Thank you for showing me that science is so much fun and thank you for always giving me the freedom in pursuing my interest. I am grateful for all the guidance from you and thank you for always supporting me whenever I needed. You are a person full of wisdom, and I have been learning so much from you and your attitude to science, science community and even life will continue inspire me. Thank you for being my mentor and thank you for being such a great mentor. Everyone else in Vale lab, past and present, for making our lab a sweet home. I would like to give my special thank to Marvin (Marvin Tanenbaum) and Nico (Nico Stuurman), two other mentors for me in the lab. I would like to thank them for helping me adapt to our lab, for all the valuable advice and for all the happiness during the time that we work together. -
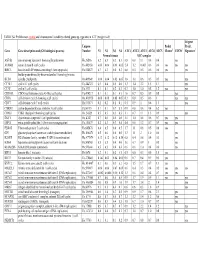
TABLE S4: Proliferation-Related and Chromosomal Instability-Related
TABLE S4: Proliferation-related and chromosomal instability-related genes up-regulated in ATC (weight <4.0) 44-gene Unigene Prolif. Prolif. Gene Gene description and (GO biological process) Number N1 N2 N3 N4 ATC1 ATC2 ATC3 ATC4 ATC5 Cluster1 CIN702 Signature3 Normal tissues ATC samples ASF1B anti-silencing function 1 homolog B (unknown) Hs.26516 0.2 0.3 0.3 0.3 0.8 0.8 1.1 0.8 0.4 yes AURKB aurora kinase B (cell cycle) Hs.442658 0.06 0.06 0.08 0.05 2.4 1.2 0.003 0.6 0.6 yes yes yes BIRC5 baculoviral IAP repeat-containing 5 (anti apoptosis) Hs.514527 0.7 0.3 0.4 0.3 0.8 0.5 0.5 0.6 0.6 yes yes budding uninhibited by benzimidazoles 1 homolog (mitotic BUB1 spindle checkpoint) Hs.469649 0.06 0.04 0.02 0.05 1.8 1.0 0.6 0.5 0.5 yes yes CCNE1 cyclin E1 (cell cycle) Hs.244723 0.5 0.4 0.5 0.6 1.3 1.4 2.2 1.5 1.1 yes CCNF cyclin F (cell cycle) Hs.1973 0.1 0.1 0.2 0.3 0.7 1.0 1.0 0.8 1.2 yes yes CDC45L CDC45 cell division cycle 45-like (cell cycle) Hs.474217 0.1 0.1 0.1 0.3 1.6 0.7 0.5 0.9 0.8 yes CDC6 cell division cycle 6 homolog (cell cycle) Hs. 405958 0.08 0.08 0.08 0.07 0.3 0.8 0.5 0.6 1 yes yes CDC7 cell division cycle 7 (cell cycle) Hs.533573 0.2 0.2 0.2 0.1 0.5 0.9 1 0.6 1.3 yes CDKN3 cyclin-dependent kinase inhibitor 3 (cell cycle) Hs.84113 0.1 0.1 0.7 0.1 0.9 0.8 0.6 0.4 6.2 yes CHEK1 CHK1 checkpoint homolog (cell cycle) Hs. -

1 AGING Supplementary Table 2
SUPPLEMENTARY TABLES Supplementary Table 1. Details of the eight domain chains of KIAA0101. Serial IDENTITY MAX IN COMP- INTERFACE ID POSITION RESOLUTION EXPERIMENT TYPE number START STOP SCORE IDENTITY LEX WITH CAVITY A 4D2G_D 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ B 4D2G_E 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ C 6EHT_D 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ D 6EHT_E 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ E 6GWS_D 41-72 41 72 100 100 3.2Å PCNA X-RAY DIFFRACTION √ F 6GWS_E 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ G 6GWS_F 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ H 6IIW_B 2-11 2 11 100 100 1.699Å UHRF1 X-RAY DIFFRACTION √ www.aging-us.com 1 AGING Supplementary Table 2. Significantly enriched gene ontology (GO) annotations (cellular components) of KIAA0101 in lung adenocarcinoma (LinkedOmics). Leading Description FDR Leading Edge Gene EdgeNum RAD51, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, CENPW, HJURP, NDC80, CDCA5, NCAPH, BUB1, ZWILCH, CENPK, KIF2C, AURKA, CENPN, TOP2A, CENPM, PLK1, ERCC6L, CDT1, CHEK1, SPAG5, CENPH, condensed 66 0 SPC24, NUP37, BLM, CENPE, BUB3, CDK2, FANCD2, CENPO, CENPF, BRCA1, DSN1, chromosome MKI67, NCAPG2, H2AFX, HMGB2, SUV39H1, CBX3, TUBG1, KNTC1, PPP1CC, SMC2, BANF1, NCAPD2, SKA2, NUP107, BRCA2, NUP85, ITGB3BP, SYCE2, TOPBP1, DMC1, SMC4, INCENP. RAD51, OIP5, CDK1, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, ESCO2, CENPW, HJURP, TTK, NDC80, CDCA5, BUB1, ZWILCH, CENPK, KIF2C, AURKA, DSCC1, CENPN, CDCA8, CENPM, PLK1, MCM6, ERCC6L, CDT1, HELLS, CHEK1, SPAG5, CENPH, PCNA, SPC24, CENPI, NUP37, FEN1, chromosomal 94 0 CENPL, BLM, KIF18A, CENPE, MCM4, BUB3, SUV39H2, MCM2, CDK2, PIF1, DNA2, region CENPO, CENPF, CHEK2, DSN1, H2AFX, MCM7, SUV39H1, MTBP, CBX3, RECQL4, KNTC1, PPP1CC, CENPP, CENPQ, PTGES3, NCAPD2, DYNLL1, SKA2, HAT1, NUP107, MCM5, MCM3, MSH2, BRCA2, NUP85, SSB, ITGB3BP, DMC1, INCENP, THOC3, XPO1, APEX1, XRCC5, KIF22, DCLRE1A, SEH1L, XRCC3, NSMCE2, RAD21. -

The Ubiquitin Conjugating Enzyme: an Important Ubiquitin Transfer Platform in Ubiquitin-Proteasome System
International Journal of Molecular Sciences Review The Ubiquitin Conjugating Enzyme: An Important Ubiquitin Transfer Platform in Ubiquitin-Proteasome System Weigang Liu 1,2, Xun Tang 2,3, Xuehong Qi 2,3, Xue Fu 2,3, Shantwana Ghimire 1,2, Rui Ma 1, Shigui Li 1, Ning Zhang 3 and Huaijun Si 1,2,3,* 1 College of Agronomy, Gansu Agricultural University, Lanzhou 730070, China; [email protected] (W.L.); [email protected] (S.G.); [email protected] (R.M.); [email protected] (S.L.) 2 Gansu Provincial Key Laboratory of Aridland Crop Science, Gansu Agricultural University, Lanzhou 730070, China; [email protected] (X.T.); [email protected] (X.Q.); [email protected] (X.F.) 3 College of Life Science and Technology, Gansu Agricultural University, Lanzhou 730070, China; [email protected] * Correspondence: [email protected]; Tel.: +86-931-7631875 Received: 3 March 2020; Accepted: 15 April 2020; Published: 21 April 2020 Abstract: Owing to a sessile lifestyle in nature, plants are routinely faced with diverse hostile environments such as various abiotic and biotic stresses, which lead to accumulation of free radicals in cells, cell damage, protein denaturation, etc., causing adverse effects to cells. During the evolution process, plants formed defense systems composed of numerous complex gene regulatory networks and signal transduction pathways to regulate and maintain the cell homeostasis. Among them, ubiquitin-proteasome pathway (UPP) is the most versatile cellular signal system as well as a powerful mechanism for regulating many aspects of the cell physiology because it removes most of the abnormal and short-lived peptides and proteins. -

Emi1 (FBXO5) (Center) Rabbit Polyclonal Antibody Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for AP51634PU-N Emi1 (FBXO5) (Center) Rabbit Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: WB Recommended Dilution: ELISA: 1/1000. Western Blot: 1/100-1/500. Reactivity: Human Host: Rabbit Isotype: Ig Clonality: Polyclonal Immunogen: KLH conjugated synthetic peptide between 191-221 amino acids from the Central region of Human FBXO5. Specificity: This antibody recognizes Human FBXO5 (Center). Formulation: PBS containing 0.09% (W/V) Sodium Azide as preservative State: Aff - Purified State: Liquid purified Ig fraction Concentration: lot specific Purification: Affinity Chromatography on Protein A Conjugation: Unconjugated Storage: Store undiluted at 2-8°C for one month or (in aliquots) at -20°C for longer. Avoid repeated freezing and thawing. Stability: Shelf life: one year from despatch. Gene Name: Homo sapiens F-box protein 5 (FBXO5), transcript variant 2 Database Link: Entrez Gene 26271 Human Q9UKT4 This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 Emi1 (FBXO5) (Center) Rabbit Polyclonal Antibody – AP51634PU-N Background: This gene encodes a member of the F-box protein family which is characterized by an approximately 40 amino acid motif, the F-box. The F-box proteins constitute one of the four subunits of the ubiquitin protein ligase complex called SCFs (SKP1-cullin-F-box), which function in phosphorylation-dependent ubiquitination. -

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body. -

Differential Gene Expression in the Skin of Xiphophorus
DIFFERENTIAL GENE EXPRESSION IN THE SKIN OF XIPHOPHORUS MACULATUS JP 163 B IN RESPONSE TO FULL SPECTRUM (10,000 K) FLUORESCENT LIGHT by Kaela Caballero, B.S. A thesis submitted to the Graduate Council of Texas State University in partial fulfillment of the requirements for the degree of Master of Science with a Major in Biochemistry August 2015 Committee Members: Ronald Walter, Chair Rachell Booth Steven Whitten COPYRIGHT by Kaela L. Caballero 2015 FAIR USE AND AUTHOR’S PERMISSION STATEMENT Fair Use This work is protected by the Copyright Laws of the United States (Public Law 94-553, section 107). Consistent with fair use as defined in the Copyright Laws, brief quotations from this material are allowed with proper acknowledgment. Use of this material for financial gain without the author’s express written permission is not allowed. Duplication Permission As the copyright holder of this work I, Kaela L. Caballero, authorize duplication of this work, in whole or in part, for educational or scholarly purposes only. DEDICATION To my parents and family, who have always supported and encouraged me, my education and my coffee addiction. And to Max, my study buddy; where you have gone, there is no need for coffee fueled late nights. Rest in peace. ACKNOWLEDGEMENTS This work could not have been accomplished without the support and mentorship that I have received throughout my life from family, teachers, mentors and friends. It is impossible to name everyone here, but I would like to use a few lines to thank those that have helped me achieve my goals. Thank you to Dr. -

Expression of a Mirna Targeting Mutated SOD1 in Astrocytes Induces Motoneuron Plasticity and Improves Neuromuscular Function in ALS Mice
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.08.425706; this version posted January 9, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Expression of a miRNA targeting mutated SOD1 in astrocytes induces motoneuron plasticity and improves neuromuscular function in ALS mice Rochat C.1, Bernard-Marissal N.1,6, Pradervand S.3, Perrin F.E.4, Raoul C.5, Aebischer P.1, Schneider B.L.1,2* 1 Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland 2 Bertarelli Platform for Gene Therapy, Ecole Polytechnique Fédérale de Lausanne (EPFL), Geneva, Switzerland 3 Genomic Technologies Facility, University of Lausanne, Lausanne, Switzerland. 4 INSERM U1198, University of Montpellier, EPHE, Place Eugène Bataillon CC105, F-34095, Montpellier, France 5 The Neuroscience Institute of Montpellier, Inserm UMR1051, Univ Montpellier, Saint Eloi Hospital, Montpellier, France 6 Aix Marseille Univ, INSERM, MMG, Marseille, France *Correspondence should be addressed to B.L.S. ([email protected]) Short title: miRNA gene therapy targeting astrocytes in ALS Study was performed in Lausanne, Switzerland Current address of corresponding author: Bernard Schneider EPFL SV PTECH PTBTG Ch. des Mines 9 CH-1202 Genève Switzerland Email: [email protected] Tel: +41 21 693 95 05 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.08.425706; this version posted January 9, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Loss of the Mammalian APC/C Activator FZR1 Shortens G1 and Lengthens S Phase but Has Little Effect on Exit from Mitosis
4208 Research Article Loss of the mammalian APC/C activator FZR1 shortens G1 and lengthens S phase but has little effect on exit from mitosis Reinhard Sigl1, Cornelia Wandke1, Veronika Rauch1, Jane Kirk2, Tim Hunt2 and Stephan Geley1,* 1Division of Molecular Pathophysiology, Biocenter, Innsbruck Medical University, Innsbruck, Austria 2Clare Hall Laboratories, Cancer Research UK, South Mimms, England, UK *Author for correspondence ([email protected]) Accepted 10 September 2009 Journal of Cell Science 122, 4208-4217 Published by The Company of Biologists 2009 doi:10.1242/jcs.054197 Summary The anaphase-promoting complex/cyclosome (APC/C) is knockdown of p53 in U2OS cells with inducible FZR1 siRNA essential for progression through mitosis. At anaphase onset, also failed to restore their proliferative capacity. Thus, the the APC/C requires the activator protein CDC20 to target proliferation defects are a direct consequence of the genetic securin and cyclin B1 for proteasome-dependent degradation, damage inflicted by loss of FZR1 function and are largely but then depends on the CDC20-related protein FZR1 (also independent of p53. In summary, mammalian FZR1 is not known as CDH1) to remain active until the onset of the next S required for the completion of mitosis, but is an important phase. To investigate the role of FZR1 in mammalian cells, we regulator of G1 phase and is required for efficient DNA used RNAi in human cell lines and conditional gene targeting replication in human and mouse somatic cells. in mouse embryonic fibroblasts. In neither case was FZR1 required for exit from mitosis, but in cells lacking FZR1, the Supplementary material available online at G1 phase was shortened and the S phase was prolonged. -

Mdm2-Mediated Ubiquitylation: P53 and Beyond
Cell Death and Differentiation (2010) 17, 93–102 & 2010 Macmillan Publishers Limited All rights reserved 1350-9047/10 $32.00 www.nature.com/cdd Review Mdm2-mediated ubiquitylation: p53 and beyond J-C Marine*,1 and G Lozano2 The really interesting genes (RING)-finger-containing oncoprotein, Mdm2, is a promising drug target for cancer therapy. A key Mdm2 function is to promote ubiquitylation and proteasomal-dependent degradation of the tumor suppressor protein p53. Recent reports provide novel important insights into Mdm2-mediated regulation of p53 and how the physical and functional interactions between these two proteins are regulated. Moreover, a p53-independent role of Mdm2 has recently been confirmed by genetic data. These advances and their potential implications for the development of new cancer therapeutic strategies form the focus of this review. Cell Death and Differentiation (2010) 17, 93–102; doi:10.1038/cdd.2009.68; published online 5 June 2009 Mdm2 is a key regulator of a variety of fundamental cellular has also emerged from recent genetic studies. These processes and a very promising drug target for cancer advances and their potential implications for the development therapy. It belongs to a large family of (really interesting of new cancer therapeutic strategies form the focus of this gene) RING-finger-containing proteins and, as most of its review. For a more detailed discussion of Mdm2 and its other members, Mdm2 functions mainly, if not exclusively, as various functions an interested reader should also consult an E3 ligase.1 It targets various substrates for mono- and/or references9–12. poly-ubiquitylation thereby regulating their activities; for instance by controlling their localization, and/or levels by The p53–Mdm2 Regulatory Feedback Loop proteasome-dependent degradation. -

A Systems-Wide Screen Identifies Substrates of the SCF Ubiquitin Ligase
RESEARCH RESOURCE PROTEOMICS A systems-wide screen identifies substrates of the SCFbTrCP ubiquitin ligase Teck Yew Low,1,2 Mao Peng,1,2* Roberto Magliozzi,3* Shabaz Mohammed,1,2† Daniele Guardavaccaro,3 Albert J. R. Heck1,2‡ Cellular proteins are degraded by the ubiquitin-proteasome system (UPS) in a precise and timely fashion. Such precision is conferred by the high substrate specificity of ubiquitin ligases. Identification of substrates of ubiquitin ligases is crucial not only to unravel the molecular mechanisms by which the UPS controls protein degradation but also for drug discovery purposes because many established UPS substrates are implicated in disease. We developed a combined bioinformatics and affinity purification– mass spectrometry (AP-MS) workflow for the system-wide identification of substrates of SCFbTrCP,a member of the SCF family of ubiquitin ligases. These ubiquitin ligases are characterized by a multi- subunit architecture typically consisting of the invariable subunits Rbx1, Cul1, and Skp1, and one of 69 F-box proteins. The F-box protein of this member of the family is bTrCP. SCFbTrCP binds, through the WD40 b repeats of TrCP, to the DpSGXX(X)pS diphosphorylated motif in its substrates. We recovered 27 pre- Downloaded from viously reported SCFbTrCP substrates, of which 22 were verified by two independent statistical proto- cols, thereby confirming the reliability of this approach. In addition to known substrates, we identified 221 proteins that contained the DpSGXX(X)pS motif and also interacted specifically with the WD40 repeats of bTrCP. Thus, with SCFbTrCP, as the example, we showed that integration of structural infor- mation, AP-MS, and degron motif mining constitutes an effective method to screen for substrates of ubiquitin ligases.