Differential Gene Expression in the Skin of Xiphophorus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytogenomic SNP Microarray - Fetal ARUP Test Code 2002366 Maternal Contamination Study Fetal Spec Fetal Cells
Patient Report |FINAL Client: Example Client ABC123 Patient: Patient, Example 123 Test Drive Salt Lake City, UT 84108 DOB 2/13/1987 UNITED STATES Gender: Female Patient Identifiers: 01234567890ABCD, 012345 Physician: Doctor, Example Visit Number (FIN): 01234567890ABCD Collection Date: 00/00/0000 00:00 Cytogenomic SNP Microarray - Fetal ARUP test code 2002366 Maternal Contamination Study Fetal Spec Fetal Cells Single fetal genotype present; no maternal cells present. Fetal and maternal samples were tested using STR markers to rule out maternal cell contamination. This result has been reviewed and approved by Maternal Specimen Yes Cytogenomic SNP Microarray - Fetal Abnormal * (Ref Interval: Normal) Test Performed: Cytogenomic SNP Microarray- Fetal (ARRAY FE) Specimen Type: Direct (uncultured) villi Indication for Testing: Patient with 46,XX,t(4;13)(p16.3;q12) (Quest: EN935475D) ----------------------------------------------------------------- ----- RESULT SUMMARY Abnormal Microarray Result (Male) Unbalanced Translocation Involving Chromosomes 4 and 13 Classification: Pathogenic 4p Terminal Deletion (Wolf-Hirschhorn syndrome) Copy number change: 4p16.3p16.2 loss Size: 5.1 Mb 13q Proximal Region Deletion Copy number change: 13q11q12.12 loss Size: 6.1 Mb ----------------------------------------------------------------- ----- RESULT DESCRIPTION This analysis showed a terminal deletion (1 copy present) involving chromosome 4 within 4p16.3p16.2 and a proximal interstitial deletion (1 copy present) involving chromosome 13 within 13q11q12.12. This -

PARSANA-DISSERTATION-2020.Pdf
DECIPHERING TRANSCRIPTIONAL PATTERNS OF GENE REGULATION: A COMPUTATIONAL APPROACH by Princy Parsana A dissertation submitted to The Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland July, 2020 © 2020 Princy Parsana All rights reserved Abstract With rapid advancements in sequencing technology, we now have the ability to sequence the entire human genome, and to quantify expression of tens of thousands of genes from hundreds of individuals. This provides an extraordinary opportunity to learn phenotype relevant genomic patterns that can improve our understanding of molecular and cellular processes underlying a trait. The high dimensional nature of genomic data presents a range of computational and statistical challenges. This dissertation presents a compilation of projects that were driven by the motivation to efficiently capture gene regulatory patterns in the human transcriptome, while addressing statistical and computational challenges that accompany this data. We attempt to address two major difficulties in this domain: a) artifacts and noise in transcriptomic data, andb) limited statistical power. First, we present our work on investigating the effect of artifactual variation in gene expression data and its impact on trans-eQTL discovery. Here we performed an in-depth analysis of diverse pre-recorded covariates and latent confounders to understand their contribution to heterogeneity in gene expression measurements. Next, we discovered 673 trans-eQTLs across 16 human tissues using v6 data from the Genotype Tissue Expression (GTEx) project. Finally, we characterized two trait-associated trans-eQTLs; one in Skeletal Muscle and another in Thyroid. Second, we present a principal component based residualization method to correct gene expression measurements prior to reconstruction of co-expression networks. -

Phosphorylation and Activation of Cell Division Cycle Associated 8 by Aurora Kinase B Plays a Significant Role in Human Lung Carcinogenesis
Research Article Phosphorylation and Activation of Cell Division Cycle Associated 8 by Aurora Kinase B Plays a Significant Role in Human Lung Carcinogenesis Satoshi Hayama,1,2 Yataro Daigo,1 Takumi Yamabuki,1 Daizaburo Hirata,1 Tatsuya Kato,1 Masaki Miyamoto,2 Tomoo Ito,3 Eiju Tsuchiya,4 Satoshi Kondo,2 andYusuke Nakamura 1 1Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, The University of Tokyo, Tokyo, Japan; Departments of 2Surgical Oncology and 3Surgical Pathology, Hokkaido University Graduate School of Medicine, Sapporo, Japan; and 4Kanagawa Cancer Center Research Institute, Kanagawa, Japan Abstract inhibitors for EGFR tyrosine kinase (i.e., gefitinib and erlotinib), Through genome-wide gene expression analysis of lung were developed and are applied in clinical practice (5). However, carcinomas, we detected in the great majority of lung cancer each of the new regimens can provide survival benefits to a small samples cotransactivation of cell division cycle associated subset of the patients (6, 7). Hence, new therapeutic strategies, such 8(CDCA8) and aurora kinase B (AURKB), which were as development of more effective molecular targeted agents considered to be components of the vertebrate chromosomal applicable to the great majority of patients with less toxicity, are passenger complex.Immunohistochemical analysis of lung eagerly awaited. cancer tissue microarrays showed that overexpression of Systematic analysis of expression levels of thousands of genes CDCA8 and AURKB was significantly associated with poor using cDNA microarrays is an effective approach for identification prognosis of lung cancer patients.AURKB directly phosphor- of unknown molecules involved in carcinogenic pathways and can ylated CDCA8 at Ser154, Ser219, Ser275, and Thr278 and seemed effectively screen candidate target molecules for development of to stabilize CDCA8 protein in cancer cells.Suppression of novel therapeutics and diagnostics. -

Evolution, Expression and Meiotic Behavior of Genes Involved in Chromosome Segregation of Monotremes
G C A T T A C G G C A T genes Article Evolution, Expression and Meiotic Behavior of Genes Involved in Chromosome Segregation of Monotremes Filip Pajpach , Linda Shearwin-Whyatt and Frank Grützner * School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia; fi[email protected] (F.P.); [email protected] (L.S.-W.) * Correspondence: [email protected] Abstract: Chromosome segregation at mitosis and meiosis is a highly dynamic and tightly regulated process that involves a large number of components. Due to the fundamental nature of chromosome segregation, many genes involved in this process are evolutionarily highly conserved, but duplica- tions and functional diversification has occurred in various lineages. In order to better understand the evolution of genes involved in chromosome segregation in mammals, we analyzed some of the key components in the basal mammalian lineage of egg-laying mammals. The chromosome passenger complex is a multiprotein complex central to chromosome segregation during both mitosis and meio- sis. It consists of survivin, borealin, inner centromere protein, and Aurora kinase B or C. We confirm the absence of Aurora kinase C in marsupials and show its absence in both platypus and echidna, which supports the current model of the evolution of Aurora kinases. High expression of AURKBC, an ancestor of AURKB and AURKC present in monotremes, suggests that this gene is performing all necessary meiotic functions in monotremes. Other genes of the chromosome passenger complex complex are present and conserved in monotremes, suggesting that their function has been preserved Citation: Pajpach, F.; in mammals. -

By Submitted in Partial Satisfaction of the Requirements for Degree of in In
Developments of Two Imaging based Technologies for Cell Biology Researches by Xiaowei Yan DISSERTATION Submitted in partial satisfaction of the requirements for degree of DOCTOR OF PHILOSOPHY in Biochemistry and Molecular Biology in the GRADUATE DIVISION of the UNIVERSITY OF CALIFORNIA, SAN FRANCISCO Approved: ______________________________________________________________________________Ronald Vale Chair ______________________________________________________________________________Jonathan Weissman ______________________________________________________________________________Orion Weiner ______________________________________________________________________________ ______________________________________________________________________________ Committee Members Copyright 2021 By Xiaowei Yan ii DEDICATION Everything happens for the best. To my family, who supported me with all their love. iii ACKNOWLEDGEMENTS The greatest joy of my PhD has been joining UCSF, working and learning with such a fantastic group of scientists. I am extremely grateful for all the support and mentorship I received and would like to thank: My mentor, Ron Vale, who is such a great and generous person. Thank you for showing me that science is so much fun and thank you for always giving me the freedom in pursuing my interest. I am grateful for all the guidance from you and thank you for always supporting me whenever I needed. You are a person full of wisdom, and I have been learning so much from you and your attitude to science, science community and even life will continue inspire me. Thank you for being my mentor and thank you for being such a great mentor. Everyone else in Vale lab, past and present, for making our lab a sweet home. I would like to give my special thank to Marvin (Marvin Tanenbaum) and Nico (Nico Stuurman), two other mentors for me in the lab. I would like to thank them for helping me adapt to our lab, for all the valuable advice and for all the happiness during the time that we work together. -

Download Paper
M BoC | ARTICLE Chromosomal passenger complex hydrodynamics suggests chaperoning of the inactive state by nucleoplasmin/nucleophosmin Mariah L. Hanleya,b, Tae Yeon Yooc, Matthew Sonnetta, Daniel J. Needlemanc,d, and Timothy J. Mitchisona,* aDepartment of Systems Biology, Harvard Medical School, Boston, MA 02114–5701; bDepartment of Chemistry, cJohn A. Paulson School of Engineering and Applied Sciences, and dDepartment of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138-2902 ABSTRACT The chromosomal passenger complex (CPC) is a conserved, essential regulator of Monitoring Editor cell division. As such, significant anti–cancer drug development efforts have been focused on Yixian Zheng targeting it, most notably by inhibiting its AURKB kinase subunit. The CPC is activated by Carnegie Institution AURKB-catalyzed autophosphorylation on multiple subunits, but how this regulates CPC in- Received: Dec 20, 2016 teractions with other mitotic proteins remains unclear. We investigated the hydrodynamic Revised: Mar 27, 2017 behavior of the CPC in Xenopus laevis egg cytosol using sucrose gradient sedimentation and Accepted: Apr 4, 2017 in HeLa cells using fluorescence correlation spectroscopy. We found that autophosphoryla- tion of the CPC decreases its sedimentation coefficient in egg cytosol and increases its diffu- sion coefficient in live cells, indicating a decrease in mass. Using immunoprecipitation coupled with mass spectrometry and immunoblots, we discovered that inactive, unphosphorylated CPC interacts with nucleophosmin/nucleoplasmin proteins, which are known to oligomerize into pentamers and decamers. Autophosphorylation of the CPC causes it to dissociate from nucleophosmin/nucleoplasmin. We propose that nucleophosmin/nucleoplasmin complexes serve as chaperones that negatively regulate the CPC and/or stabilize its inactive form, pre- venting CPC autophosphorylation and recruitment to chromatin and microtubules in mitosis. -
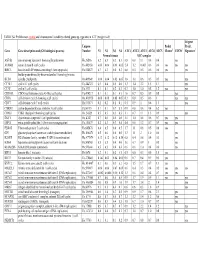
TABLE S4: Proliferation-Related and Chromosomal Instability-Related
TABLE S4: Proliferation-related and chromosomal instability-related genes up-regulated in ATC (weight <4.0) 44-gene Unigene Prolif. Prolif. Gene Gene description and (GO biological process) Number N1 N2 N3 N4 ATC1 ATC2 ATC3 ATC4 ATC5 Cluster1 CIN702 Signature3 Normal tissues ATC samples ASF1B anti-silencing function 1 homolog B (unknown) Hs.26516 0.2 0.3 0.3 0.3 0.8 0.8 1.1 0.8 0.4 yes AURKB aurora kinase B (cell cycle) Hs.442658 0.06 0.06 0.08 0.05 2.4 1.2 0.003 0.6 0.6 yes yes yes BIRC5 baculoviral IAP repeat-containing 5 (anti apoptosis) Hs.514527 0.7 0.3 0.4 0.3 0.8 0.5 0.5 0.6 0.6 yes yes budding uninhibited by benzimidazoles 1 homolog (mitotic BUB1 spindle checkpoint) Hs.469649 0.06 0.04 0.02 0.05 1.8 1.0 0.6 0.5 0.5 yes yes CCNE1 cyclin E1 (cell cycle) Hs.244723 0.5 0.4 0.5 0.6 1.3 1.4 2.2 1.5 1.1 yes CCNF cyclin F (cell cycle) Hs.1973 0.1 0.1 0.2 0.3 0.7 1.0 1.0 0.8 1.2 yes yes CDC45L CDC45 cell division cycle 45-like (cell cycle) Hs.474217 0.1 0.1 0.1 0.3 1.6 0.7 0.5 0.9 0.8 yes CDC6 cell division cycle 6 homolog (cell cycle) Hs. 405958 0.08 0.08 0.08 0.07 0.3 0.8 0.5 0.6 1 yes yes CDC7 cell division cycle 7 (cell cycle) Hs.533573 0.2 0.2 0.2 0.1 0.5 0.9 1 0.6 1.3 yes CDKN3 cyclin-dependent kinase inhibitor 3 (cell cycle) Hs.84113 0.1 0.1 0.7 0.1 0.9 0.8 0.6 0.4 6.2 yes CHEK1 CHK1 checkpoint homolog (cell cycle) Hs. -

Mir-190 Enhances Endocrine Therapy Sensitivity by Regulating SOX9
Yu et al. Journal of Experimental & Clinical Cancer Research (2019) 38:22 https://doi.org/10.1186/s13046-019-1039-9 RESEARCH Open Access miR-190 enhances endocrine therapy sensitivity by regulating SOX9 expression in breast cancer Yue Yu1,2,3,4, Wen Yin1,2,3,4, Zhi-Hao Yu1,2,3,4, Yan-Jun Zhou1,2,3,4, Jiang-Rui Chi1,2,3,4, Jie Ge1,2,3,4 and Xu-Chen Cao1,2,3,4* Abstract Background: Breast cancer is the most common cancer among women worldwide, and approximately 70% of breast cancers are hormone receptor-positive and express estrogen receptor-α (ERα) or/and progesterone receptor. Therapies targeting ERα have been successfully used in patients with ERα+ breast cancer. However, intrinsic or acquired resistance to anti-estrogen therapy presents a major challenge. The Wnt/β-catenin signaling pathway regulates various processes that are important for cancer progression, and emerging evidences have shown a close interaction between Wnt/β-catenin and ERα signaling. miR-190 is also involved in ER signaling and our previous study indicated that miR-190 suppresses breast cancer metastasis. Methods: The effect of miR-190 on breast cancer anti-estrogen sensitivity was investigated both in vitro and in vivo. The protein expression levels and localization were analyzed by western blotting and immunofluorescence, respectively. Chromatin immunoprecipitation and dual-luciferase reporter assays were used to validate the regulation of the zinc-finger E-box binding homeobox 1/ ERα-miR-190-SRY-related high mobility group box 9 (ZEB1/ERα-miR-190-SOX9) axis. Results: miR-190 increased the anti-estrogen sensitivity of breast cancer cells both in vitro and in vivo. -

1 AGING Supplementary Table 2
SUPPLEMENTARY TABLES Supplementary Table 1. Details of the eight domain chains of KIAA0101. Serial IDENTITY MAX IN COMP- INTERFACE ID POSITION RESOLUTION EXPERIMENT TYPE number START STOP SCORE IDENTITY LEX WITH CAVITY A 4D2G_D 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ B 4D2G_E 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ C 6EHT_D 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ D 6EHT_E 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ E 6GWS_D 41-72 41 72 100 100 3.2Å PCNA X-RAY DIFFRACTION √ F 6GWS_E 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ G 6GWS_F 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ H 6IIW_B 2-11 2 11 100 100 1.699Å UHRF1 X-RAY DIFFRACTION √ www.aging-us.com 1 AGING Supplementary Table 2. Significantly enriched gene ontology (GO) annotations (cellular components) of KIAA0101 in lung adenocarcinoma (LinkedOmics). Leading Description FDR Leading Edge Gene EdgeNum RAD51, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, CENPW, HJURP, NDC80, CDCA5, NCAPH, BUB1, ZWILCH, CENPK, KIF2C, AURKA, CENPN, TOP2A, CENPM, PLK1, ERCC6L, CDT1, CHEK1, SPAG5, CENPH, condensed 66 0 SPC24, NUP37, BLM, CENPE, BUB3, CDK2, FANCD2, CENPO, CENPF, BRCA1, DSN1, chromosome MKI67, NCAPG2, H2AFX, HMGB2, SUV39H1, CBX3, TUBG1, KNTC1, PPP1CC, SMC2, BANF1, NCAPD2, SKA2, NUP107, BRCA2, NUP85, ITGB3BP, SYCE2, TOPBP1, DMC1, SMC4, INCENP. RAD51, OIP5, CDK1, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, ESCO2, CENPW, HJURP, TTK, NDC80, CDCA5, BUB1, ZWILCH, CENPK, KIF2C, AURKA, DSCC1, CENPN, CDCA8, CENPM, PLK1, MCM6, ERCC6L, CDT1, HELLS, CHEK1, SPAG5, CENPH, PCNA, SPC24, CENPI, NUP37, FEN1, chromosomal 94 0 CENPL, BLM, KIF18A, CENPE, MCM4, BUB3, SUV39H2, MCM2, CDK2, PIF1, DNA2, region CENPO, CENPF, CHEK2, DSN1, H2AFX, MCM7, SUV39H1, MTBP, CBX3, RECQL4, KNTC1, PPP1CC, CENPP, CENPQ, PTGES3, NCAPD2, DYNLL1, SKA2, HAT1, NUP107, MCM5, MCM3, MSH2, BRCA2, NUP85, SSB, ITGB3BP, DMC1, INCENP, THOC3, XPO1, APEX1, XRCC5, KIF22, DCLRE1A, SEH1L, XRCC3, NSMCE2, RAD21. -

Comprehensive Identification of Survival-Associated Genes for Cancers
bioRxiv preprint doi: https://doi.org/10.1101/526285; this version posted January 22, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Comprehensive identification of survival-associated genes for cancers Hongde Liu1*, Kun Luo2, Huamei Li1, Xiao Sun1 1State Key Laboratory of Bioelectronics, School of Biological Science & Medical Engineering, Southeast University, Nanjing 210096, China 2Department of Neurosurgery, Xinjiang Evidence-Based Medicine Research Institute, First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, China *The corresponding author: Hongde Liu, e-mail: [email protected] (HDL). Authors’ e-mails: Hongde Liu: [email protected] (HDL); Kun Luo: [email protected] (KL); Huamei Li: [email protected](HML); Xiao Sun: [email protected](XS). Highlights: ● The number of possible prognostic and diagnostic genes for cancers; ● A list of independent prognostic genes for each cancer; ● The universal prognostic genes mainly function in the spindle assembly checkpoint; ● Statistical links between mutated pathways and prognostic genes. 1 bioRxiv preprint doi: https://doi.org/10.1101/526285; this version posted January 22, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract: Prognostic signature is important in estimating cancer risk, subtyping cancer, and planning treatment. -

Emi1 (FBXO5) (Center) Rabbit Polyclonal Antibody Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for AP51634PU-N Emi1 (FBXO5) (Center) Rabbit Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: WB Recommended Dilution: ELISA: 1/1000. Western Blot: 1/100-1/500. Reactivity: Human Host: Rabbit Isotype: Ig Clonality: Polyclonal Immunogen: KLH conjugated synthetic peptide between 191-221 amino acids from the Central region of Human FBXO5. Specificity: This antibody recognizes Human FBXO5 (Center). Formulation: PBS containing 0.09% (W/V) Sodium Azide as preservative State: Aff - Purified State: Liquid purified Ig fraction Concentration: lot specific Purification: Affinity Chromatography on Protein A Conjugation: Unconjugated Storage: Store undiluted at 2-8°C for one month or (in aliquots) at -20°C for longer. Avoid repeated freezing and thawing. Stability: Shelf life: one year from despatch. Gene Name: Homo sapiens F-box protein 5 (FBXO5), transcript variant 2 Database Link: Entrez Gene 26271 Human Q9UKT4 This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 Emi1 (FBXO5) (Center) Rabbit Polyclonal Antibody – AP51634PU-N Background: This gene encodes a member of the F-box protein family which is characterized by an approximately 40 amino acid motif, the F-box. The F-box proteins constitute one of the four subunits of the ubiquitin protein ligase complex called SCFs (SKP1-cullin-F-box), which function in phosphorylation-dependent ubiquitination. -

Associated 16P11.2 Deletion in Drosophila Melanogaster
ARTICLE DOI: 10.1038/s41467-018-04882-6 OPEN Pervasive genetic interactions modulate neurodevelopmental defects of the autism- associated 16p11.2 deletion in Drosophila melanogaster Janani Iyer1, Mayanglambam Dhruba Singh1, Matthew Jensen1,2, Payal Patel 1, Lucilla Pizzo1, Emily Huber1, Haley Koerselman3, Alexis T. Weiner 1, Paola Lepanto4, Komal Vadodaria1, Alexis Kubina1, Qingyu Wang 1,2, Abigail Talbert1, Sneha Yennawar1, Jose Badano 4, J. Robert Manak3,5, Melissa M. Rolls1, Arjun Krishnan6,7 & 1234567890():,; Santhosh Girirajan 1,2,8 As opposed to syndromic CNVs caused by single genes, extensive phenotypic heterogeneity in variably-expressive CNVs complicates disease gene discovery and functional evaluation. Here, we propose a complex interaction model for pathogenicity of the autism-associated 16p11.2 deletion, where CNV genes interact with each other in conserved pathways to modulate expression of the phenotype. Using multiple quantitative methods in Drosophila RNAi lines, we identify a range of neurodevelopmental phenotypes for knockdown of indi- vidual 16p11.2 homologs in different tissues. We test 565 pairwise knockdowns in the developing eye, and identify 24 interactions between pairs of 16p11.2 homologs and 46 interactions between 16p11.2 homologs and neurodevelopmental genes that suppress or enhance cell proliferation phenotypes compared to one-hit knockdowns. These interac- tions within cell proliferation pathways are also enriched in a human brain-specific network, providing translational relevance in humans. Our study indicates a role for pervasive genetic interactions within CNVs towards cellular and developmental phenotypes. 1 Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, PA 16802, USA. 2 Bioinformatics and Genomics Program, The Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA 16802, USA.