Michigan COVID-19 Vaccination Interim Prioritization Guidance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
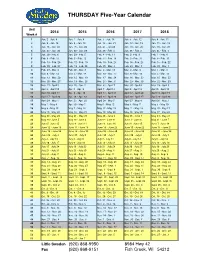
Thursday Calendar 20142018
THURSDAY Five-Year Calendar Unit 2014 20152016 2017 2018 Week # 1 Jan. 2 - Jan. 9 Jan. 1 - Jan. 8 Jan. 7 - Jan. 14 Jan. 5 - Jan. 12 Jan. 4 - Jan. 11 2 Jan. 9 - Jan. 16 Jan. 8 - Jan. 15 Jan. 14 - Jan. 21 Jan. 12 - Jan. 19 Jan. 11 - Jan. 18 3 Jan. 16 - Jan. 23 Jan. 15 - Jan. 22 Jan. 21 - Jan 28 Jan. 19 - Jan. 26 Jan. 18 - Jan. 25 4 Jan. 23 - Jan. 30 Jan. 22 - Jan. 29 Jan. 28 - Feb. 4 Jan. 26 - Feb. 2 Jan. 25 - Feb. 1 5 Jan. 30 - Feb. 6 Jan. 29 - Feb. 5 Feb. 4 - Feb. 11 Feb. 2- Feb. 9 Feb. 1 - Feb. 8 6 Feb. 6 - Feb. 13 Feb. 5 - Feb. 12 Feb. 11 - Feb. 18 Feb. 9 - Feb. 16 Feb. 8 - Feb. 15 7 Feb. 13 - Feb. 20 Feb. 12 - Feb. 19 Feb. 18 - Feb. 25 Feb. 16 - Feb. 23 Feb. 15 - Feb. 22 8 Feb. 20 - Feb. 27 Feb. 19 - Feb. 26 Feb. 25 - Mar. 3 Feb. 23 - Mar. 2 Feb. 22 - Mar. 1 9 Feb. 27 - Mar. 6 Feb. 26 - Mar. 5 Mar. 3 - Mar. 10 Mar. 2 - Mar. 9 Mar. 1 - Mar. 8 10 Mar. 6 - Mar. 13 Mar. 5 - Mar. 12 Mar. 10 - Mar. 17 Mar. 9 - Mar. 16 Mar. 5 - Mar. 15 11 Mar. 13 - Mar. 20 Mar. 12 - Mar. 19 Mar. 17 - Mar. 24 Mar. 16 - Mar. 23 Mar. 15 - Mar. 22 12 Mar. 20 - Mar. 27 Mar. 19 - Mar. 26 Mar. 24 - Mar. 31 Mar. 23 - Mar. 30 Mar. 22 - Mar. -

(March 1, 1919) We Hereby Declare That Korea Is an Independent
Primary Source Document with Questions (DBQs) DECLARATION OF INDEPENDENCE (MARCH 1, 1919) Introduction The first decade of Japanese colonial rule in Korea was one of harsh repression. In 1919, however, a group of prominent Koreans secretly prepared a Declaration of Independence rejecting Japanese rule and its presumptions and, on March 1, read the document aloud in Seoul’s Pagoda Park. Months of largely peaceful, nationwide demonstrations followed, ultimately involving more than one million Koreans. Japanese authorities responded with force, resulting in thousands of deaths and an even larger number of arrests before the independence movement was put down. In the aftermath, however, Japanese government officials sought to defuse the situation by allowing for a time greater Korean cultural and political expression, though calls for outright political action against colonial rule were still forbidden. The March 1 movement has remained a touchstone for Korean nationalist sentiment up to the present. Document Excerpts with Questions (Longer selection follows this section) From Sources of Korean Tradition, edited by Yŏng‐ho Ch’oe, Peter H. Lee, and Wm. Theodore de Bary, vol. 2 (New York: Columbia University Press, 2000), 337‐339. © 2000 Columbia University Press. Reproduced with the permission of the publisher. All rights reserved. Declaration of Independence (March 1, 1919) We hereby declare that Korea is an independent state and that Korean are a self‑ governing people. We proclaim it to the nations of the world in affirmation of the principle of the equality of all nations, and we proclaim it to our posterity, preserving in perpetuity the right of national survival. We make this declaration on the strength of five thousand years of history as an expression of the devotion and loyalty of twenty million people. -

Vaccination Certificate Information Version: 21 July 2021
معلومات عن شهادة التطعيم، بالعربية 中文疫苗接种凭证信息 Informations sur le certificat de vaccination en FRANÇAIS Информация о сертификате вакцинации на РУССКОМ ЯЗЫКЕ Información sobre el Certificado de Vacunación en ESPAÑOL UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME VACCINATION CERTIFICATE INFORMATION VERSION: 21 JULY 2021 ABOUT THE UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME The UN is committed to ensuring the protection of its personnel. Tasked by the Secretary-General, the Department of Operational Support (DOS) is leading a coordinated, UN-system-wide effort to ensure the availability of vaccine to UN personnel, their dependents and implementing partners. The roll-out of the UN System-wide COVID-19 Vaccination Programme (the “Programme”) for UN personnel will provide a significant boost to the ability of personnel to stay and deliver on the Organization's mandates, to support beneficiaries in the communities they serve, and to contribute to our on-going work to recover better together from the pandemic. Information about the Programme is available at: https://www.un.org/en/coronavirus/vaccination ABOUT THE VACCINATION CERTIFICATE Upon administration of the requisite vaccine dosage, a certificate of vaccination is generated through the Programme’s registration platform (the “Platform”). The certificate generated by the Platform uses a standardized format, similar to the one used by the WHO in its “International Certificate of Vaccination or Prophylaxis” (Yellow) vaccination booklet. Note: This certificate may not dispense with travel restrictions put in place by the country(ies) of destination. As the nature of the pandemic continues to rapidly evolve, it is highly advisable to check the latest travel regulations. -

Global Dynamics of a Vaccination Model for Infectious Diseases with Asymptomatic Carriers
MATHEMATICAL BIOSCIENCES doi:10.3934/mbe.2016019 AND ENGINEERING Volume 13, Number 4, August 2016 pp. 813{840 GLOBAL DYNAMICS OF A VACCINATION MODEL FOR INFECTIOUS DISEASES WITH ASYMPTOMATIC CARRIERS Martin Luther Mann Manyombe1;2 and Joseph Mbang1;2 Department of Mathematics, Faculty of Science University of Yaounde 1, P.O. Box 812 Yaounde, Cameroon 1;2;3; Jean Lubuma and Berge Tsanou ∗ Department of Mathematics and Applied Mathematics University of Pretoria, Pretoria 0002, South Africa (Communicated by Abba Gumel) Abstract. In this paper, an epidemic model is investigated for infectious dis- eases that can be transmitted through both the infectious individuals and the asymptomatic carriers (i.e., infected individuals who are contagious but do not show any disease symptoms). We propose a dose-structured vaccination model with multiple transmission pathways. Based on the range of the explic- itly computed basic reproduction number, we prove the global stability of the disease-free when this threshold number is less or equal to the unity. Moreover, whenever it is greater than one, the existence of the unique endemic equilibrium is shown and its global stability is established for the case where the changes of displaying the disease symptoms are independent of the vulnerable classes. Further, the model is shown to exhibit a transcritical bifurcation with the unit basic reproduction number being the bifurcation parameter. The impacts of the asymptomatic carriers and the effectiveness of vaccination on the disease transmission are discussed through through the local and the global sensitivity analyses of the basic reproduction number. Finally, a case study of hepatitis B virus disease (HBV) is considered, with the numerical simulations presented to support the analytical results. -

GOOGLE LLC V. ORACLE AMERICA, INC
(Slip Opinion) OCTOBER TERM, 2020 1 Syllabus NOTE: Where it is feasible, a syllabus (headnote) will be released, as is being done in connection with this case, at the time the opinion is issued. The syllabus constitutes no part of the opinion of the Court but has been prepared by the Reporter of Decisions for the convenience of the reader. See United States v. Detroit Timber & Lumber Co., 200 U. S. 321, 337. SUPREME COURT OF THE UNITED STATES Syllabus GOOGLE LLC v. ORACLE AMERICA, INC. CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE FEDERAL CIRCUIT No. 18–956. Argued October 7, 2020—Decided April 5, 2021 Oracle America, Inc., owns a copyright in Java SE, a computer platform that uses the popular Java computer programming language. In 2005, Google acquired Android and sought to build a new software platform for mobile devices. To allow the millions of programmers familiar with the Java programming language to work with its new Android plat- form, Google copied roughly 11,500 lines of code from the Java SE pro- gram. The copied lines are part of a tool called an Application Pro- gramming Interface (API). An API allows programmers to call upon prewritten computing tasks for use in their own programs. Over the course of protracted litigation, the lower courts have considered (1) whether Java SE’s owner could copyright the copied lines from the API, and (2) if so, whether Google’s copying constituted a permissible “fair use” of that material freeing Google from copyright liability. In the proceedings below, the Federal Circuit held that the copied lines are copyrightable. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

Monday, April 5, 2021
Monday, April 5, 2021 WHERE WE ARE TODAY 3 April 5, 2021 OUR PROGRESS ON VACCINATIONS 4 # DC residents partially or fully vaccinated January 1 5,846 February 1 40,839 March 1 74,811 April 1 162,669 (Apr 2) Data source: DC Health, using received data through 4/2/2021. Data subject to change. April 5, 2021 5 We have more work to do to get all residents vaccinated. Make sure you’re signed up to get your COVID-19 shot. Go to: Call: vaccinate.dc.gov 1-855-363-0333 April 5, 2021 PRE-REGISTRATION SNAPSHOT 6 Approximately 190,621 people have pre-registered for a vaccination appointment and are awaiting an appointment. April 5, 2021 7 If you pre-registered for a vaccination appointment but already received your vaccine elsewhere you can email [email protected] to remove yourself from the pre-registration list. April 5, 2021 NEW AND UPCOMING CLINICS 8 This week, the vaccine clinic at the Convention Center will expand through a partnership with Safeway. Appointment invitations will be sent on April 6. On Friday, April 9, a new high-capacity vaccination site will open at Arena Stage. The site will be operated in partnership with DC Health and MedStar Health. Appointments will be made through vaccinate.dc.gov and the District’s call center. April 5, 2021 THE PATH FORWARD FIVE FACTORS DETERMINING AN ACTIVITY’S RE-OPEN POTENTIAL 10 1. MASKS - Can and is the activity completed with or without wearing a mask? Highest Risk Unmasked, Indoors, Long Time, Very Dense 2. -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -

AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Statements Contained in This Release As the Result of New Information Or Future Events Or Developments
Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant NEW YORK and MAINZ, GERMANY, July 8, 2021 — As part of Pfizer’s and BioNTech’s continued efforts to stay ahead of the virus causing COVID-19 and circulating mutations, the companies are providing an update on their comprehensive booster strategy. Pfizer and BioNTech have seen encouraging data in the ongoing booster trial of a third dose of the current BNT162b2 vaccine. Initial data from the study demonstrate that a booster dose given 6 months after the second dose has a consistent tolerability profile while eliciting high neutralization titers against the wild type and the Beta variant, which are 5 to 10 times higher than after two primary doses. The companies expect to publish more definitive data soon as well as in a peer-reviewed journal and plan to submit the data to the FDA, EMA and other regulatory authorities in the coming weeks. In addition, data from a recent Nature paper demonstrate that immune sera obtained shortly after dose 2 of the primary two dose series of BNT162b2 have strong neutralization titers against the Delta variant (B.1.617.2 lineage) in laboratory tests. The companies anticipate that a third dose will boost those antibody titers even higher, similar to how the third dose performs for the Beta variant (B.1.351). Pfizer and BioNTech are conducting preclinical and clinical tests to confirm this hypothesis. While Pfizer and BioNTech believe a third dose of BNT162b2 has the potential to preserve the highest levels of protective efficacy against all currently known variants including Delta, the companies are remaining vigilant and are developing an updated version of the Pfizer-BioNTech COVID-19 vaccine that targets the full spike protein of the Delta variant. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February